REVIEW ARTICLE
Applied Molecular Biology Laboratory (AMBL), School of Life Sciences, Jawaharlal Nehru University, New Delhi, India.
*Corresponding Author: Prof. Pramod Kumar Yadava
Citation: Pramod Kumar Yadava2*, Zika Virus, A Tool to Fight Against Glioblastoma, Brain Science and Neurosurgery vol 1(1). Immunology Advances and Infectious Pathways (IAIP) DOI: 10.1875 iaip.2024/001.
Copyright: © (2024), Pramod Kumar Yadava2*, this is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 20, 2024 | Accepted: March 09, 2024 | Published: March 18, 2024
Abstract
Glioblastoma multiforme (GBM) is a deadly human cancer. It occurs in the brain cells or in spinal cord. GBM stem cells are reported to be resistant to treatment and reappearance is unpreventable. GBM stem cells are the target of Zika virus (ZIKV). The virus ensures the survival of mice having gliomas. ZIKV has been reported as an oncolytic virus targeting GBM cells selectively. Here, we will discuss the immunological aspect of protection against GBM mediated by ZIKV. When ZIKV was introduced into the brain, the recruitment of CD8+ T and myeloid cells to the tumor microenvironment was augmented. CD8+ T cells were needed for ZIKV-mediated tumor clearance. Here we will also discuss the role of cellular gasdermin D (GSDMD) in efficient killing of a human GBM cell line that was promoted by ZIKV infection. The ZIKV protease cleaves specifically human GSDMD for activating caspase-independent pyroptosis; as a consequence both the naive neighboring as well as viral protease-harboring cells are harmed.
Keywords: Glioblastoma multiforme (GBM), Glioblastoma Stem Cells (GSC), Zika virus (ZIKV), Gasdermin D (GSDMD), SRY (sex determining region Y)-box 2 (SOX2).
Introduction
GBM is the most invasive primary brain tumor. Almost all patients succumb to death within two years of detection [1]. Surgery, temozolomide chemotherapy, radiation therapy and use of adjuvants more recently comprise standard treatment [2]. In spite of maximal treatment, the recurrence of most GBMs within 6 months was reported at that time when no curative or standard treatment persists. The outcomes of poor patients from GBM are multifaceted that included the existence of GBM stem cells (GSCs), which are reported to be resistant to chemotherapy as well as radiation therapy [3–5] and feeble antitumor immunological responses [6–11]. Cancer is reported to be one of the major causes of death in this world. The World Health Organization categorized GBM as a grade IV glioma among different brain tumors due to its malignant nature [12]. This invasive tumor sustains cell proliferation, escape from immune system, and resistance to drugs because GBM stem cells are reported to be self-renewing, multipotent, and resistant to apoptosis [13, 14].
ZIKV is reported to be transmitted by Aedes mosquitoes primarily. These mosquitoes usually bite during day time. Most people infected with ZIKV hardly develop symptoms. Those who develop symptoms typically suffer from rash, conjunctivitis, fever, joint and muscle pain, headache and malaise that continues for 2–7 days. If ZIKV infection occurs during pregnancy, it leads to the birth of infants with microcephaly and other inherited malformations in addition to miscarriage and premature birth. Myelitis, neuropathy and Guillain-Barre syndrome in adults and children are reported to be associated with ZIKV infection. Although from 2017 onwards the cases of ZIKV disease declined globally. The transmission is still prevalent at low levels in some American countries [15, 16].
For ZIKV infection no specific treatment is available. People having symptoms of rash, joint pain or fever should drink plenty of fluids, take sufficient rest and can be treated with analgesics or antipyretics. Pregnant women infected with ZIKV should immediately seek medical treatment [17].
2. ZIKV Genetic Material & Oncolysis:
In 2007 for the first time the whole genome of MR 766, the African prototype ZIKV strain, was sequenced. The ZIKV arbovirus belongs to the Flavivirus genus of the Flaviviridae family [18]. The ZIKV genome contains a 10.8-kb positive-sense, single-stranded RNA molecule, which consists of an 5′ untranslated region (UTR) of around 100 nt length, one single open reading frame (ORF) of around 10 kb, and 3′ UTR of around 420 nt. A single polyprotein encoded by the ORF is processed further into the capsid C, the precursor membrane prM, the envelope protein E and seven nonstructural proteins viz., NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 [19] (Fig. 1).
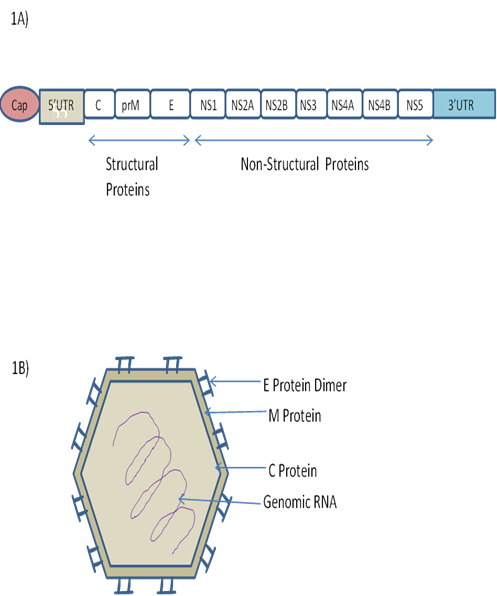
Fig.1: Zika Virus. (A) Zika Virus Genome. (B) Zika Virus Structure [19].
The evolution of the ZIKV genome in a rapid manner was reported to account for the current ZIKV pandemic with unique Zika disease. The immune system activated by ZIKV promotes the activation and proliferation of glial cells and induces apoptotic cell death. The development of inflammation has critical contribution to Zika disease [20].
An oncolytic virus would be considered as a desirable candidate for cancer treatment if the virus destroys the cancer cells selectively without harming the normal healthy cells [21, 22]. Irrespective of the genetic material, DNA or RNA, several viruses were categorized as oncolytic viruses. This includes Newcastle disease virus, adenovirus, herpes simplex virus 1, poliovirus, vaccinia virus, parvovirus and reovirus [23-25]. Recently, the causative agent of microcephaly in the fetus, ZIKV was categorized as an oncolytic virus as it infects and destroys the GBM stem cells preferentially and probably does not injure adult neurons [26, 27].
3. Viral Neurotropism:
Although ZIKV was reported to kill both the pediatric and adult brain cancer cells, adult GBMs were studied with preference for illustrating the oncolytic mechanisms that is T cells requirement for increasing the efficacy [28-30]. The ZIKV neurotropism was possibly due to the specific expression of αvβ5 and SOX2 on the surface of GBM stem cells [31]. The ZIKV receptor tyrosine kinase AXL expression makes GBM cells greatly permissive to ZIKV; thereby the killing effects. Nonetheless the viral receptor expression is a necessity for virus infection but this hardly guarantees the killing [32].
Integrin αvβ5 is reported to be proangiogenic member of broader RGD-binding integrin family. They are the primary receptors utilized by animal cells for binding to the extracellular matrix. The heterodimers work as transmembrane proteins [33]. The characterization of the distribution of integrins in human cancers is of huge interest. At present there is little knowledge regarding the αvβ5 expression in gastric cancers, mainly due to dearth of antibodies appropriate for utilization on formalin-fixed and paraffin-embedded (FFPE) tissues [34].
SRY (sex determining region Y)-box 2 is termed as SOX2, a transcription factor, essential for maintenance of pluripotency or self-renewal of embryonic stem cells that are undifferentiated. Sox2 plays a critical function for maintaining neural and embryonic stem cells [31].
An in-depth examination is needed to know how ZIKV destroys GBM cells after causing infection for assessing the plus and minus points of accurate virotherapy. Among several kinds of virus-mediated cell death, recently pyroptosis was interpreted as protein-mediated cellular destruction of gasdermin family accompanied by inflammatory responses distinguishable mechanistically from apoptosis [35, 36].
4. Mode of Replication:
Virions that are attached to the receptors on cell surfaces enter the cells subsequently by receptor-driven endocytosis and are taken into the vesicles coated with clathrin inside the cells. Conformational changes are triggered by endosome acidification, viral membrane getting fused with endosome membrane, followed by particle disassembly and release of viral genomic RNA into the cytoplasm from viral nucleocapsid [37, 38].
After the release of positive-stranded genomic RNA from nucleocapsid into cytoplasm, the RdRp synthesizes the negative-strand genomic RNA using the positive-strand genome as template [39]. On the endoplasmic reticulum surface, new viral positive-strand genomes are synthesized using the negative-strand RNA. The newly synthesized genomes are translated further into viral polyproteins by host cell machinery. The ssRNA viral genome then undergoes translation into a single polyprotein, which is processed further co-translationally as well as post-translationally by cellular and viral proteases. This cleavage is reported to produce three structural proteins (C, prM and E) as well as seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) [40].
As reported earlier the viral genome replication starts with the synthesis of negative-strand RNA, and serves further as a template for the positive-strand genomic RNA synthesis. The assembly of virus occurs in the surface of endoplasmic reticulum (ER) by the process of budding. The virus particles, yet to be matured, traverse through the trans-Golgi system along the host secretory pathway. The virion maturation takes place at trans-Golgi system and subsequently released by the process of exocytosis from the cell [41, 42].
The replication process of ZIKV is depicted in the Fig. 2.
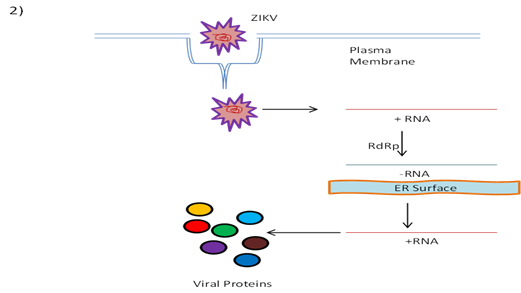
Fig.2: A schematic representation of ZIKV replication. Positive stranded RNA is encoded into a negative strand, followed by a positive strand before encoding proteins [40].
5. Immunology of Oncolytic ZIKV:
ZIKV is reported to augment the infiltration of CD8+ T cells into the tumor site. Solid tumors with little quantity of T cell infiltration normally do not get any benefit from immune checkpoint blockade treatment [43-45] (Fig. 3).
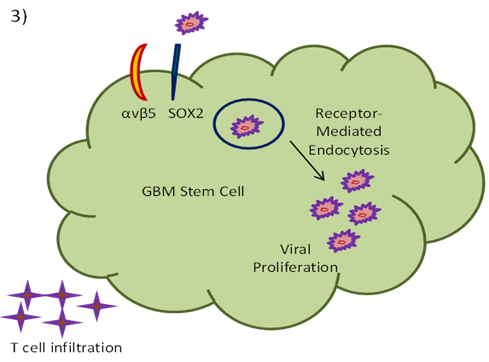
Fig. 3: T cell infiltration at GBM tumor site after ZIKV infection [45].
In this context oncolytic virotherapy is a fruitful treatment option. This is because the inflammation induced by the virus is able to increase the effectiveness of checkpoint blockade treatment. While monotherapy using anti–PD-1 antibody, improved tumor survival to a limited degree, when administered together with ZIKV, tumor survival is enhanced. PD-1 is a checkpoint protein on T cells. A vivid study in humans revealed that Talimogene laherparepvec, T-VEC together with anti–PD-1 immunotherapy had a bearable safety profile in melanoma treatment, and the combination was reported to have greater effectiveness against melanoma compared to checkpoint blockade anti–PD-1 antibody monotherapy or T-VEC treatment [46]. Because of successfulness of the inhibitors of immune checkpoints in other types of cancers and their probable supplementary effects with several oncolytic viruses, many combinations of virus/antibody are recently being probed in clinical trials. This involves a proceeding clinical trial at phase II stage with pembrolizumab (anti–PD-1) combined with an oncolytic adenovirus (DNX-2401) for patients suffering from recurrent GBM (ClinicalTrials.gov NCT02798406) [47, 48].
An analogous conjugation with ZIKV will be worth following. In addition to this, future studies should be conducted in nonresponders to PD-1 blockade and ZIKV combination therapy. This may point out the mechanisms of resistance, as for example immune infiltration reduction surrounding the tumor, depletion of tumor antigens, or other processes leading to T cell exhaustion or anergy [29].
ZIKV treatment also enhanced the response of myeloid cells that are associated with tumors in the tumor site, particularly the microglia and monocyte populations. Macrophage subsets associated to tumors may contribute to presentation of antigens as well as the anticancer immune cycle. They may boost up growth of tumor cells and arrest an immune response [49-51]. Further studies will surely make it clear what rebalancing and skewing of myeloid cells ZIKV treatment ushers in.
6. GSDMD-mediated cell death by ZIKV protease:
ZIKV is reported to be an oncolytic virus as it destroys human GBM by GSDMD activation mediated by viral protease. This cleavage is species-specific. This suggests that human GSDMD transgenic mice models, i.e., humanized animals, never a simple GBM mice model would provide assuring experimental outcomes of preclinical trials on ZIKV oncotherapy [52].
GSDMD, a novel biomarker to evaluate the progression of cancer due to an elevated level of protein expression in glioma and it is reported to be connected with significant longevity of GBM patients [53]. Pyroptosis mediated by GSDMD accompanies the pro-inflammatory cytokine, IL-1β release that helps in supporting immune response against carcinoma mediated by the T helper 1 cells specific to the particular tumor [54, 55]. The mature IL-1β release by conventional pyroptosis is due to caspase-4/5/11 or caspase-1- mediated cleavage of GSDMD that intern ensures the release of GSDMD-N to perforate the plasma membrane [56]. Because caspase-3-mediated GSDMD-N cleavage negatively regulates the process, infection-elicited caspases may manipulate the pyroptosis activated by ZIKV protease [57].
Overexpression of E protein of ZIKV alone is adequate for suppressing proliferation of cells as well as in inducing caspase-mediated programmed cell death [58]. In the 3’ untranslated region of viral genome deletion of 10-nt generates a live-attenuated strain of ZIKV both in vivo and in vitro. This strain was suggested as a vaccine candidate for treatment against ZIKV as well as to treat malignant GBM [59, 60].
Among several cytokines and proteins that are secreted following ZIKV infection, the release of GSDMD-N is a matter of interest. Upon release GSDMD-N may cause harm to the uninfected cancer cells for expanding the therapeutic effects as well as to the normal healthy tissues for generating side effects. Taking into consideration that GBM is not a liquid tumor but a solid one, with secretion of GSDMD-N locally and capability of ZIKV replication, a little dosage of ZIKV would be enough for the treatment. The extracellular GSDMD-N is reported to aid patients having brain tumor who are vulnerable to bacterial infection as GSDMD peptide is antibacterial [61].
7. Future Perspectives:
Whether the caspase profiles induced by ZIKV or particular caspase inhibitors have any effect on the therapeutic outcomes of anti-tumor treatment using ZIKV is to be investigated further. Virulence factors that contribute to cell death induced by ZIKV required to be detected for providing the basis of applying ZIKV. Therefore, to do away with any unpredictability regarding other ZIKV constituents, single-round infectious particles or nanoparticles harboring GSDMD-N or the ZIKV protease should be safe and sound in comparison to the live virus to treat cancer [52].
To date, clinical trials based on ZIKV virotherapy has not yet been conducted for cancer treatment. Coexistence of pros and cons can cause complications in the virotherapy. An appropriate animal model to study ZIKV therapy for treating human GBM is yet to be developed. It was demonstrated that activation of GSDMD by ZIKV protease is associated with the prognosis as well as management of ZIKV virotherapy. The genetic background of GSDMD could be utilized for screening positive responders to conduct virotherapy. When ZIKV infection is out-of-control, for stopping the therapy our target should be ZIKV protease. Nonetheless, the small molecules that are reported to regulate the activation of GSDMD might perform to terminate the virotherapy that is not depended on the activity of ZIKV protease. Until a profound understanding regarding the mechanisms that underlie cell lysis induced by ZIKV is obtained, this study will provide a base of reference for preclinical assessment to predict therapeutic outcomes as well as the probable influence of effects of treatment that are combined with medicines targeting oligomerization of GSDMD or activity of caspases [52].
8. Limitations:
It was demonstrated in the previous work that the replication of ZIKV is hugely self-limited to Glioblastoma Stem Cells, partly due to their intrinsically enervated innate immune response as well as important integrin signaling molecules expression that ensure infection, the most important concern is safety [62, 63].
It was reported that the protease 3C of the enterovirus causes to stop pyroptosis by cleaving GSDMD-N further, so oncotherapy using ZIKV would prove to be complicated if the coinfecting microbes are able to antagonize activation of GSDMD [64].
9. Conclusion:
In a nutshell we can comment that ZIKV will become an emerging tool for the treatment of Glioblastoma. Natural property of ZIKV will be exploited to destroy the unnatural outcomes of Glioblastoma.
References
-
Stupp R, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466.
Publisher | Google Scholor -
Stupp R, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316.
Publisher | Google Scholor -
Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–1217
Publisher | Google Scholor -
Patel AP, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401.
Publisher | Google Scholor -
Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760.
Publisher | Google Scholor -
El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8(3):234–243.
Publisher | Google Scholor -
Han S, et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110(10):2560–2568.
Publisher | Google Scholor -
Raychaudhuri B, et al. Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. J Neurooncol. 2015;122(2):293–301.
Publisher | Google Scholor -
Raychaudhuri B, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–599.
Publisher | Google Scholor -
Gielen PR, et al. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro Oncol. 2016;18(9):1253–1264.
Publisher | Google Scholor -
Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res. 2018;24(16):3792–3802.
Publisher | Google Scholor -
Stoyanov, G.S., Lyutfi, E., Georgieva, R., Georgiev, R., Dzhenkov, D.L., Petkova, L.,Ivanov, B.D., Kaprelyan, A., and Ghenev, P. (2022). Reclassification of glioblastoma multiforme according to the 2021 World health organization classification of central nervous system tumors: a single Institution report and practical significance. Cureus 14, e21822.
Publisher | Google Scholor -
Bao, S., Wu, Q., McLendon, R.E., Hao, Y., Shi, Q., Hjelmeland, A.B., Dewhirst, M.W.,Bigner, D.D., and Rich, J.N. (2006). Glioma stem cells promote radioresistance bypreferential activation of the DNA damage response. Nature 444, 756–760.
Publisher | Google Scholor -
Alvarado, A.G., Thiagarajan, P.S., Mulkearns-Hubert, E.E., Silver, D.J., Hale, J.S.,Alban, T.J., Turaga, S.M., Jarrar, A., Reizes, O., Longworth, M.S., et al. (2017). Glioblastoma cancer stem cells evade innate immune suppression of self-renewal through reduced TLR4 expression. Cell Stem Cell 20, 450–461.e4.
Publisher | Google Scholor -
de Araújo TVB, Ximenes RA de A, Miranda-Filho D de B, et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: Final report of a case-control study. Lancet Infect Dis. 3099(17)30727-2.
Publisher | Google Scholor -
Krauer F, Riesen M, Reveiz L, et al. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review. PLoS Med. 2017;14(1). doi:10.1371/journal.pmed.10022.
Publisher | Google Scholor -
Musso D, Ko AI, Baud D. Zika Virus Infection – After the Pandemic. N Engl J Med. 2019;381(15). doi:10.1056/nejmra1808246.
Publisher | Google Scholor -
Huang Y-JS, Higgs S, Horne KE et al. Flavivirus-mosquito interactions. Viruses 2014; 6: 4703–4730.
Publisher | Google Scholor -
Petersen LR, Jamieson DJ, Powers AM et al. Zika virus. N Engl J Med 2016; 374: 1552–1563.
Publisher | Google Scholor -
Wang A, Thurmond S, Islas L, Hui K, and Hai R. Zika virus genome biology and molecular pathogenesis. Emerg Microbes Infect. 2017 Mar; 6(3): e13.
Publisher | Google Scholor -
Kaufman, H.L., Kohlhapp, F.J., and Zloza, A. (2015). Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 14, 642–662.
Publisher | Google Scholor -
Peruzzi, P., and Chiocca, E.A. (2018). Viruses in cancer therapy - from benchwarmersto quarterbacks. Nat. Rev. Clin. Oncol. 15, 657–658.
Publisher | Google Scholor -
Choudhury, S.R., Hudry, E., Maguire, C.A., Sena-Esteves, M., Breakefield, X.O., and Grandi, P. (2017). Viral vectors for therapy of neurologic diseases. Neuropharmacology 120, 63–80.
Publisher | Google Scholor -
Fukuhara, H., Ino, Y., and Todo, T. (2016). Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 107, 1373–1379.
Publisher | Google Scholor -
Foreman, P.M., Friedman, G.K., Cassady, K.A., and Markert, J.M. (2017). Oncolytic virotherapy for the treatment of malignant glioma. Neurotherapeutics 14, 333–344.
Publisher | Google Scholor -
Araujo, A.Q.C., Silva, M.T.T., and Araujo, A.P.Q.C. (2016). Zika virus-associated neurological disorders: a review. Brain. 139, 2122–2130
Publisher | Google Scholor -
Parra, B., Lizarazo, J., Jiménez-Arango, J.A., Zea-Vera, A.F., González-Manrique, G., Vargas, J., Angarita, J.A., Zuñiga, G., Lopez-Gonzalez, R., Beltran, C.L., et al. (2016). Guillain-barre syndrome associated with Zika virus infection in Colombia. N. Engl. J. Med. 375, 1513–1523.
Publisher | Google Scholor -
Kaid, C., Goulart, E., Caires-Júnior, L.C., Araujo, B.H.S., Soares-Schanoski, A., Bueno,H.M.S., Telles-Silva, K.A., Astray, R.M., Assoni, A.F., Júnior, A.F.R., et al. (2018). Zika virus selectively kills aggressive human embryonal CNS tumor cells in vitro and in vivo. Cancer Res. 78, 3363–3374.
Publisher | Google Scholor -
Nair, S., Mazzoccoli, L., Jash, A., Govero, J., Bais, S.S., Hu, T., Fontes-Garfias, C.R.,Shan, C., Okada, H., Shresta, S., et al. (2021). Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight 6, e144619.
Publisher | Google Scholor -
Chen, L., Zhou, C., Chen, Q., Shang, J., Liu, Z., Guo, Y., Li, C., Wang, H., Ye, Q., Li, X., et al. (2022). Oncolytic Zika virus promotes intratumoral T cell infiltration and improves immunotherapy efficacy in glioblastoma. Mol. Ther. Oncolytics 24, 522–534.
Publisher | Google Scholor -
Zhu, Z., Mesci, P., Bernatchez, J.A., Gimple, R.C., Wang, X., Schafer, S.T., Wettersten, H.I., Beck, S., Clark, A.E., Wu, Q., et al. (2020). Zika virus targets glioblastoma stem cells through a SOX2-Integrin alphavbeta5 Axis. Cell Stem Cell 26, 187–204.e10.
Publisher | Google Scholor -
Zwernik, S.D., Adams, B.H., Raymond, D.A., Warner, C.M., Kassam, A.B., Rovin, R.A., and Akhtar, P. (2021). AXL receptor is required for Zika virus strain MR-766 infection in human glioblastoma cell lines. Mol. Ther. Oncolytics 23, 447–457.
Publisher | Google Scholor -
Lippa RA, Barrett J, Pal S, Rowedder JE, Murphy JA, Barrett TN. Discovery of the first potent and selective αvβ5 integrin inhibitor based on an amide-containing core. Eur J Med Chem. 2020 Dec 15;208:112719. doi: 10.1016/j.ejmech.2020.112719. Epub 2020 Aug 25.
Publisher | Google Scholor -
Goodman S, Grote HJ, Wilm C. Matched rabbit monoclonal antibodies against αv-series integrins reveal a novel αvβ3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors. Biol Open. 2012;1:329–340. doi: 10.1242/bio.2012364.
Publisher | Google Scholor -
Aglietti, R.A., and Dueber, E.C. (2017). Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends Immunol. 38, 261–271. 36. Broz, P., Pelegrín, P., and Shao, F. (2020). The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157.
Publisher | Google Scholor -
Broz, P., Pelegrín, P., and Shao, F. (2020). The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157.
Publisher | Google Scholor -
Shi, Y.; Dai, L.; Song, H.; Gao, G.F. Structures of Zika Virus E & NS1: Relations with Virus Infection and Host Immune Responses. Front. Immunol. 2018, 1062, 77–87.
Publisher | Google Scholor -
Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688.
Publisher | Google Scholor -
Bernatchez, J.A.; Tran, L.T.; Li, J.; Luan, Y.; Siqueira-Neto, J.L.; Li, R. Drugs for the Treatment of Zika Virus Infection. J. Med. Chem. 2020, 63, 470–489.
Publisher | Google Scholor -
Ngono, A.E.; Shresta, S. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308.
Publisher | Google Scholor -
Cox, B.D.; Stanton, R.A.; Schinazi, R.F. Predicting Zika virus structural biology: Challenges and opportunities for interven-tion. Antivir. Chem. Chemother. 2015, 24, 118–126.
Publisher | Google Scholor -
Van Leur, S.W.; Heunis, T.; Munnur, D.; Sanyal, S. Pathogenesis and virulence of flavivirus infections. Virulence 2021, 12, 2814–2838.
Publisher | Google Scholor -
Peng D, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249–253.
Publisher | Google Scholor -
Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235.
Publisher | Google Scholor -
Tang H, et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;30(3):500.
Publisher | Google Scholor -
Ribas A, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell.2017;170(6):1109–1119.e10.
Publisher | Google Scholor -
LaRocca CJ, Warner SG. Oncolytic viruses and checkpoint inhibitors: combination therapy in clinical trials. Clin Transl Med.2018;7(1):35.
Publisher | Google Scholor -
Chen CY, Hutzen B, Wedekind MF, Cripe TP. Oncolytic virus and PD-1/PD-L1 blockade combination therapy. Oncolytic Virother.2018;7:65–77
Publisher | Google Scholor -
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment.Cancers (Basel). 2014;6(3):1670–1690.
Publisher | Google Scholor -
Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24.
Publisher | Google Scholor -
Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med.2013;19(10):1264–1272.
Publisher | Google Scholor -
Kao Y-T, Wang H-I,1 Shie C-T, Lin C-F, Lai MMC, and Yu C-Y. Zika virus cleaves GSDMD to disseminate prognosticable and controllable oncolysis in a human glioblastoma cell model. Molecular Therapy: Oncolytics Vol. 28 March 2023.
Publisher | Google Scholor -
Liu, J., Gao, L., Zhu, X., Geng, R., Tao, X., Xu, H., and Chen, Z. (2021). Gasdermin Dis a novel prognostic biomarker and relates to TMZ response in glioblastoma. Cancers (Basel) 13, 5620
Publisher | Google Scholor -
He, W.T., Wan, H., Hu, L., Chen, P., Wang, X., Huang, Z., Yang, Z.H., Zhong, C.Q., and Han, J. (2015). Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 25, 1285–1298.
Publisher | Google Scholor -
Haabeth, O.A.W., Lorvik, K.B., Yagita, H., Bogen, B., and Corthay, A. (2016). Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology 5, e1039763.
Publisher | Google Scholor -
Qiu, S., Liu, J., and Xing, F. (2017). ’Hints’ in the killer protein gasdermin D: unveiling the secrets of gasdermins driving cell death. Cell Death Differ. 24, 588–596.
Publisher | Google Scholor -
Taabazuing, C.Y., Okondo, M.C., and Bachovchin, D.A. (2017). Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages.Cell Chem. Biol. 24, 507–514.e4.
Publisher | Google Scholor -
Liu, J., Li, Q., Li, X., Qiu, Z., Li, A., Liang, W., Chen, H., Cai, X., Chen, X., Duan, X., et al. (2018). Zika virus envelope protein induces G2/M cell cycle arrest and apoptosis via an Intrinsic cell death signaling pathway in neuroendocrine PC12 cells. Int. J. Biol. Sci. 14, 1099–1108.
Publisher | Google Scholor -
Shan, C., Muruato, A.E., Nunes, B.T.D., Luo, H., Xie, X., Medeiros, D.B.A., Wakamiya, M., Tesh, R.B., Barrett, A.D., Wang, T., et al. (2017). A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat.Med. 23, 763–767.
Publisher | Google Scholor -
Chen, Q., Wu, J., Ye, Q., Ma, F., Zhu, Q., Wu, Y., Shan, C., Xie, X., Li, D., Zhan, X., et al. (2019). Treatment of human glioblastoma with a live attenuated Zika virus vaccine candidate. mBio 10, 004333–e519.
Publisher | Google Scholor -
Kuang, S., Zheng, J., Yang, H., Li, S., Duan, S., Shen, Y., Ji, C., Gan, J., Xu, X.W., and Li, J. (2017). Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc. Natl. Acad. Sci. USA 114, 10642–10647.
Publisher | Google Scholor -
Zhu Z, et al. Zika virus has oncolytic activity against glioblastoma stem cells. J Exp Med. 2017;214(10):2843–2857.
Publisher | Google Scholor -
Zhu Z, et al. Zika virus targets glioblastoma stem cells through a SOX2-integrin αvβ5 axis. Cell Stem Cell. 2020;26(2):187–204.e10.
Publisher | Google Scholor -
Lei, X., Zhang, Z., Xiao, X., Qi, J., He, B., and Wang, J. (2017). Enterovirus 71 inhibits pyroptosis through cleavage of gasdermin D. J. Virol. 91, 010699–e1117.
Publisher | Google Scholor
