RESEARCH ARTICLE
- Anupam Chanda 1
Packaging and PolymerScience Technologist (PG), India, BioxytranInc, MA, Boston,USA.
*Corresponding Author: Anupam Chanda ,Packaging andPolymerScienceTechnologist(PG), India,BioxytranInc, MA,Boston, USA.
Citation: Anupam Chanda, Sustainable Packaging in Microgravity for Injectables1(1). Biomedical Studies and Clinical Evaluations (BSCE)1(1), DOI: https://doi.org/10.64347/3064-7037/003
Copyright: © ( 2024) Anupam Chanda, this is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 11, 2024 | Accepted: April 05, 2024 | Published: April 22, 2024
Abstract
We all know world is really looking for sustainable packaging in order to protect the environment. Microgravity environment is quite new for human beings so right from the beginning our aim has to be every innovation should be sustainable in that critical environment. From MOON to MARS and other planets we need to develop all kinds of injectable devices according to the respective planets environment and those are too critical. Few areas are playing vital roles like high Radiations, extremeTemperatures, variations of Gravity in different locations in one particular planet.
Keywords: Microgravity, Sustainable packaging, Injectable drugs, Space medicine, Eco-friendly packaging, Space pharmaceuticals, Zero-gravity packaging
Introduction
Sand on the MOON

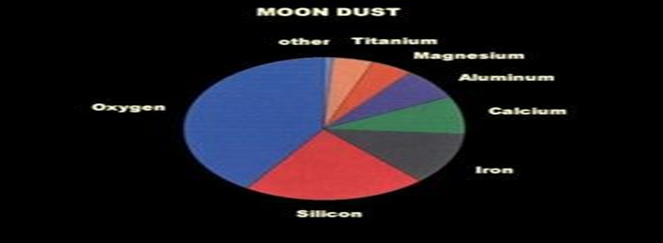
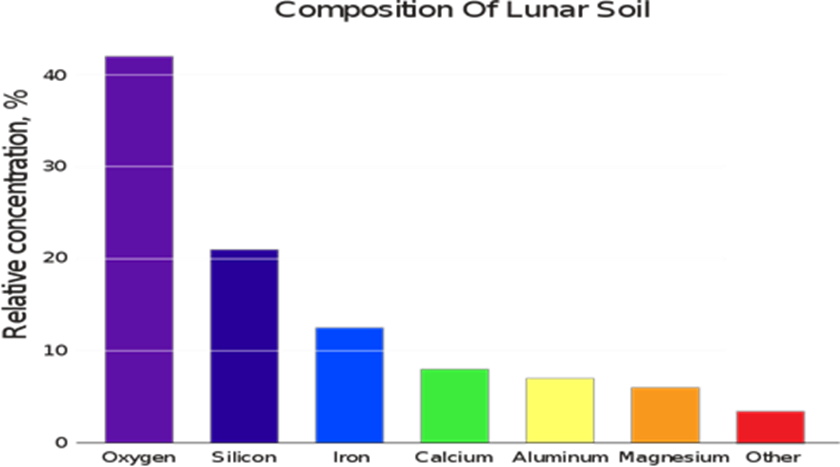
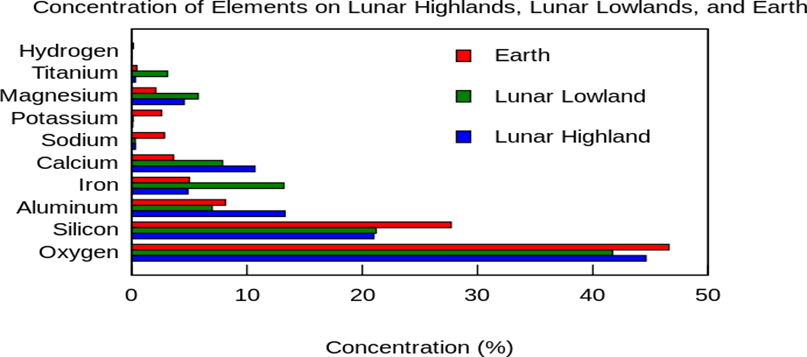
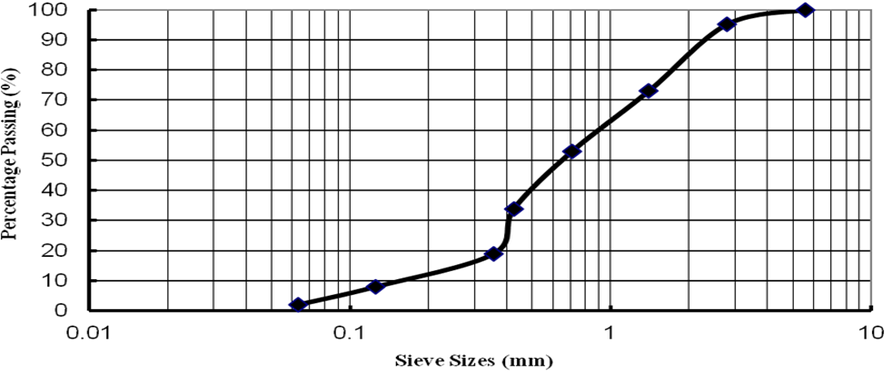
Lunar Glass: There are several reasons why glass made from lunar sand is better quality compare to Earth and those are more suitable to use for “Injectable products” for Pharma and sustainable in the Microgravity environment.
Advantages in MOON sand:
a)No atmosphere: Since there is no O2 and other interactive gasses so there is no chance to melt the glass. This prevents oxidation; Chemical reactions that can lead to impurities in the glass. There is no chance to form Air bubblesduring manufacturing of glass and inside the product as well.
b)Low gravity: This hugely influencefor formation of glass.
c)Exceptionally high Vacuum: It helps a lot during the formation of glass.
d) Purity: Achieve optical properties surpassing conventional production constraints, opening up new markets and product categories.
Autoinjector: This will manufacture from Aluminum not from Polymersin Lunar surfacesince petroleum not yet invented in MOON and MARS. Syringeswill be made in glass. Going to manufacture allmedical devices in MOON and MARS surface. This is the most logical predictions





Radiation effecton Lunar Surfaceand probable Solutionsto protect injectableproducts
Three kindsof common coatingsin solar systemsincluding the matt black painting,the black chrome and the black nickel-chrome coating were used on the absorber tube of a parabolic trough collector (PTC). The results showed that the black chrome coating with 98% purity had the highest absorption in the ultraviolet and visible range and the matt black paint had the lowest thermal conductivity.
This has been measured an average total radiation absorbeddose rate in silicon of 13.2 ± 1 μGy/hourand a neutral particle dose rate of 3.1
± 0.5 μGy/hour.
Many pharmaceutical productsare heat labileare being sterilized by gamma irradiation or at least being investigated for compatibility with this mode of sterilization. Furthermore, many powders used in the pharmaceutical industry either as active pharmaceutical ingredients or pharmaceutical adjuncts, are heavily contaminated with microorganisms because of their naturalsource, thus presenting a health hazard to the consumer. Frequently they do not withstand heating processes to reduce the initial microbial load, and so, a low radiation dose (less than 10 kGy) may be sufficient to reduce the bioburden by severalorders of magnitude.
Radiation effectobserved in followingfew Products: Amoxicillin sodium
The effectsof gamma irradiation at ambient temperature at a dose of 25 kGy on the stability of potassium clavulanate, amoxicillin sodium and their combination as powders were investigated . A decrease in purity and increase in degradation products up to 30 days after the irradiation were evaluated by reversed phase HPLC. A comparison between unirradiated and irradiated amoxicillin sodium, performed within 24 h following the irradiation process, showed no significant changes.
Clarithromycin
The effect of ionizing radiation on clarithromycin powder was investigated. HPLC analysis confirms its stability at 2 and 5 kGy radiation doses with no observed degradation products. However, at 25 kGy, the antimicrobial activity was reduced by 1.27%, and an unacceptable increase of a single impuritywas observed.
Diclofenac sodium
The influenceof gamma irradiation (up to 25 kGy)on the physicochemical properties of the NSAIDs, naproxen sodium and diclofenac sodium, when incorporated in PLGA microspheres. Drug loading of irradiated and non-irradiated microspheres was essentially the same. A significant difference was noticed, however, between particle sizes of the irradiated and non-irradiated formulations. Inrelease studies, the amount of active substance released from PLGA microspheres
Enzymes and proteins
The effect of gamma irradiation (25 kGy) on peptide-containing hydrophilic PLGA microspheres, showed that on the basis of HPLC analysis, the peptide content of the microspheres was lowered. In- vivo evaluation, however,of the non-irradiated and the 15 kGy irradiated microspheres showed no marked differences.
Carbohydrates
The effects of irradiation on various carbohydrates both in the solid phase and in aqueous solution. A method for radiation sterilization of certain sugars, particularly aqueous dextrose solution, by gamma irradiating over an extended time period of not less
Anticancer drugs
Eelectron beam irradiation of the anticancers, flutamide, ifosfamide and aminoglutethimide in microcrystalline form, two speciesidentified in flutamidewere assigned to a more stable tertiarycarbon-centred radical, and a less stable aryl radical or nitrogen- centred radical cation. Two components were found in if osfamide with one being the radical formed on the loss of a chlorineatom.
References
-
Heiken; Vanniman & French (1991). Lunar Sourcebook. Cambridge University Press. pp. 756. ISBN 978- 0-521-33444-0.
Publisher | Google Scholor -
^
Publisher | Google Scholor -
^ Stubbs, Timothy J.; Richard R. Vondrak & William M. Farrell (2005).
Publisher | Google Scholor
