RESEARCH ARTICLE
- DUNGA KINGSLEY EXCEL 1
- CHINAKWE ETIENNE 2
- IBRAHIM MARIAM ELEOJO 2
- OFOEGBU NNAMDI JUDE 1
- OKORO CHINYERE IHUARULAM 3
- NNODIM JOHNKENNEDY 4
1Department Of Medical Laboratory Science, College of Medicine and Health Sciences, Rhema University, Aba Abia State
2Department of Microbiology Kingsley Ozumba Mbadiwe University, Ideato, Imo State.
3 Department of Microbiology/Parasitology Federal Teaching Hospital, Owerri.
4.Department of Medical Laboratory Science Imo State University Owerri
*Corresponding Author: 1DUNGA KINGSLEY EXCEL
Citation: 1DUNGA KINGSLEY EXCEL SEROPREVALENCE OF HEPATITIS A VIRUS AMONG PREGNANT WOMEN IN IDEATO SOUTH LGA, IMO STATE, Maternity and Reproductive Health Sciences, vol 1(2). DOI: https://doi.org/10.64347/3064-7096/MRHS.013
Copyright: © 2024, Dr. 1DUNGA KINGSLEY EXCEL, this is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: November 18, 2024 | Accepted: November 20, 2024 | Published: December 04, 2024
Abstract
Hepatitis A is a disease that can spread during pregnancy and is preventable with a vaccine. In Ideato South LGA of Imo State, this study looked into the frequency of hepatitis A virus (HAV) antibodies among expectant mothers.A total of 125 blood samples were randomly drawn from pregnant patients who visited nearby clinics, hospitals, and maternity centers. The samples were then sent to a laboratory for screening. According to the study, the age group with the highest frequency of IgG antibodies was 19–29 years old (3.2%), whereas the age group with the highest prevalence of IgM antibodies was 30-39 years old (2.4%). Health professionals and those with university education also had higher prevalence of IgG antibodies, but people with only a secondary education had higher prevalence of IgM antibodies. These results highlight the necessity of individualized prenatal care and immunization programs for expectant mothers. Our knowledge of how occupational and socioeconomic factors can affect illness susceptibility is further enhanced by the study's assessment of these variables. In order to eliminate differences in HAV prevalence and guarantee that all pregnant women have access to efficient preventative treatments, this information can assist direct public health activities. Overall, this study adds to a more thorough understanding of the dynamics of infectious diseases in the pregnant population and has direct implications for enhancing the health of mothers and their offspring. The results can be utilized to improve immunization and prenatal care programs, as well as to guide public health initiatives that try to shield expectant mothers against HAV infection.
Keywords: seroprevalence, virus, pregnant women, Ideato South LGA, Imo State
Introduction
The hepatitis viruses are a family of viruses that cause hepatitis in people worldwide. Hepatitis is an inflammation of the liver that is characterized by the presence of inflammatory cells in the tissue of the organ, according to Jacobsen et al. (2018).Blood and other bodily fluids carry the hepatitis virus, which is transferred from host to host. Although there may be few or no symptoms at first, jaundice, anorexia (loss of appetite), and malaise are frequently the results. If the illness persists for longer than six months, it is considered chronic hepatitis. The majority of hepatitis cases globally are caused by a class of viruses known as the hepatitis viruses, although other causes include autoimmune disorders, toxins (such as alcohol, some drugs, and certain plants), and infections. The hepatitis virus is spread from person to person through bodily fluids such as blood. The most common ways that people become infected with the virus are through blood transfusions and blood products where blood-borne viruses are not screened for, medical or dental procedures in nations where equipment is not properly sterilized, mother-to-child transmission during childbirth, sexual transmission (in the case of hepatitis B), sharing injecting equipment, sharing straws, notes, etc. for snorting cocaine (cocaine is especially alkaline and corrosive), and sharing razors, toothbrushes, or other sharp objects.The five most concerned group members have been arranged alphabetically based on their high risk of illness and death, as well as their tendency to cause outbreaks and the spread of epidemics. We have hepatitis types A, B, C, D, and E.
Feinstone and associates provided a characterization of the hepatitis A virus (HAV). The hepatitis A virus (HAV) is the cause of hepatitis A, a vaccine-preventable illness (VPD). According to Wang (2015), the virus is a positive-sense RNA virus that is a member of the genus Herpavirus and family Picornaviridae. It is a small, non-enveloped, icosahedral particle with a diameter of 27 nm. According to de Jong et al. (2007), the main ways that the hepatitis A virus spreads from person to person through the faecal-oral route are through the consumption of contaminated food or water and/or contact with infected individuals.Measurements of particular antibodies, such as immunoglobulin class M (IgM) and immunoglobin class G (IgG) anti-HAV antibodies, are used to evaluate the immunological response triggered by HAV infection. Following an acute infection, anti-HAV IgM antibodies can be found, and within three to six months, antibody titres often drop to nil (Ottjj et al., 2012). Anti-HAV IgG antibodies, on the other hand, develop two to three months after infection and last for a long time, providing protection against further infections.
Worldwide, HAV infections are common, but they are more common in low-income and developing nations (Jacobsen et al., 2018). Because early childhood exposure to HAV is so common, there are almost no at-risk adults in sub-Saharan Africa and South Asia. Latin America, the Middle East, North Africa, Eastern Europe, and middle-class Asian regions are all home to intermediate endemicity. Stronger economies, such those in Western Europe and the United States, have lower rates of HAV infection, but their nonimmune adult population is far more susceptible to contracting the virus than people in lower-income nations (Havekar et al.,2015).When it comes to HAV, there is a paradoxical effect in nations where economic mobility is evident.The majority of HAV infections that happen in underdeveloped nations do not show any symptoms and are not clinically noticeable, most likely due to incomplete immunity in endemic areas. On the other hand, infections in industrialized nations are typically associated with severe hepatitis and jaundice, particularly in adults and adolescents.With 1.4 million cases reported annually worldwide, hepatitis A virus (HAV) infection is linked to considerable morbidity and mortality worldwide (WHO 2016).
However, following the introduction of the HAV immunization in 1995, the prevalence has decreased by 95% in the United States; in 2013, the prevalence was 0.6 per 100,000 population.The primary means of human-to-human transmission of HAV through the faecal-oral route is ingestion of contaminated food or water and/or contact with infected individuals.Daycare centers are frequently linked to the development of HAV because transmission from children to their parents occurs frequently (Kleven et al., 2010).Food service employees who neglect to properly wash their hands and disinfect their hands after a defecation are frequently the source of HAV from contaminated food and water (Schwarz et al., 2008).Because the virus is difficult to remove from fruit and vegetable surfaces, fresh and frozen food produce may be a contributing factor in the spread of HAV infection (Di Cola et al., 2021).
According to Ahmad et al. (2018), contaminated water can result from both contained and epidemic infection, depending on the cause (inadequate chlorination or inadequate irrigation infrastructure).According to Foster et al. (2017), the virus can also spread following organ transplantation. Rarely seen parenteral transmission is caused by transient viremia following first acquisition (Hettmann et al., 2017). Men having sex with men (MSM), visiting an endemic area, and intravenous drug use are risk factors in affluent nations (Tanaka et al., 2019). There are still developed nations with areas where HAV is endemic, as there are Native American tribes in the western states of the United States. When HAV epidemics happen, unsanitary environments are frequently to blame. In both developing and industrialized nations, insufficient sewage disposal and water contamination are frequently linked to outbreaks (Bizri et al., 2018). According to Purpari et al. (2018), outbreaks in affluent countries are frequently connected to a source of tainted food or water.In one study, it was discovered that hepatitis A virus (HAV) infection during pregnancy is linked to high rates of gestational complications and preterm labor, despite being rare (incidence of 1 per 1000 pregnancies). Stronger links were noted for HAV infection during the second and third trimesters (Elinav et al., 2006).Pregnancy and acute HAV infection are uncommon. Therefore, it is challenging to determine the occurrence throughout pregnancy (Rac et al., 2014).Fever and hypoalbuminemia are indicators of a more aggressive course of the illness.The majority of newborns whose moms had HAV infection were unaffected and had normal transaminase and antibody levels.One in ten pregnant women have acute hepatitis A virus infection. According to Chaudhry et al. (2015), the disease has a 0.3% to 0.6 mortality rate and is primarily self-limited. In industrialized countries, the disease's course during pregnancy is largely similar to that of patients who are not pregnant, with good outcomes for both the mother and the fetus. Patients with HAV who are pregnant are able to give birth vaginally, and breastfeeding is not prohibited (Daudi et al., 2012).Although there is little information available, acute HAV infection during pregnancy may increase the risk of preterm labor, placental abruption, and premature rupture of the membranes (Chaudry et al., 2015). Although cases of acute maternal HAV infection resulting to fetal liver injury and MTCT have been reported, acute liver failure due to HAV infection is rarely observed in pregnancy (Casey et al., 2020).
Although serologic testing by looking for anti-HAV immunoglobulin M (IgM) antibodies is done in high-risk patients suspected of having acute HAV infection, routine HAV screening is not advised. Immunoglobulin and the HAV vaccine are safe to use while pregnant.
The San Diego outbreak, which involved 590 confirmed cases of genotype 1b HAV in San Diego, California, between November 22, 2016, and June 21, 2018, attracted attention from throughout the world. The majority of cases involved boys or males, and MSM, injectable drug use, and homelessness were significant risk factors. Hepatitis B virus (HBV) co-infection occurred in about 5% of cases while hepatitis C virus co-infection occurred in about 17% of cases. The epidemiology of HAV outbreaks may be changing from contaminated food and water to poor sanitation centered around homelessness, overcrowding, and injection drug use, as seen by the San Diego outbreak and, more recently, an outbreak in Michigan (Zeng et al., 2021).With over 4,000 cases and 43 fatalities, the current Kentucky outbreak is showing the same pattern. Patients who use narcotics and do not have permanent housing, as was the situation in San Diego, are mostly responsible for the outbreak's spread. The main reasons this outbreak is currently the biggest and deadliest in US history are believed to be the difficulties associated with hepatitis A vaccination in rural Kentucky and the lack of funds and resources available to obtain vaccine.
Pregnancy-related hepatitis A has become a leading cause of death for fetuses and has been associated with a higher risk of complications for moms. One of the most common viral liver diseases worldwide, hepatitis A is caused by the hepatitis A virus, a small non-enveloped RNA virus (HAV). Nigeria is one of the countries with an endemic HAV infection. There are 3.8 cases recorded for per 10,000 individuals. It's possible that many of these people are ignorant of the infection and ignore to seek the appropriate medical attention, which can have negative effects like liver disease, cirrhosis, and hepatocellular cancer. Similarly, participating pregnant women represent a serious health risk to both society and the fetus.
Materials and Methods
Design of the study
The random sampling research design was used in this study
Area of study
Different hospitals, Maternities and clinics located within the local government Area, Ideato South, Imo State, Nigeria, served as the sites of this study. Before collecting all the samples, consent was derived from all the participants orally.
Population of the study
A total of 125 women made up the study population, and their serums were gathered and examined for the presence of the Hepatitis A virus.
Sample and sampling techniques
There were 125 pregnant women in the study's population, and all of them were selected randomly from the study sites visited
Instruments for data collection
HAV Gold Rapid Screen Test Strip
(For the qualitative detection of HAV antibodies in serum/ plasma and whole blood).
The HAV IGM test on cassette is a rapid chromatographic immunoassay for the qualitative detection of IgM antibodies to the hepatitis A virus (HAV) in serum or plasma and whole blood.
Sample Collection and Preparation
Fingers tick Specimens (Whole Blood)
The area to be lanced was cleaned with an alcohol swab. The end of the fingertip was squeezed and pierced with a sterile lancet. The first blood was cleaned was wiped away with sterile gauze. A micropipette was used to obtain 100µℓ of fresh blood, which was added to the sample well.
Plasma
A certified phlebotomist had collected whole blood into a purple, blue, green top collection tube (containing ETDA, citrate, or heparin, respectively) by venipuncture. The plasma had been separated by centrifugation. The plasma had been carefully withdrawn for treatment or labeled and stored at 2-8℃ for up to two weeks. The plasma may be frozen at 20℃ for up to a year.
Serum
Having had a certified phlebotomist collect whole blood into a red top collection tube (containing no anticoagulants) by venepuncture, I allowed the blood to clot. The serum had been separated by centrifugation. I carefully withdrew the serum for testing and stored it at 2-8℃ Serum may be frozen at 200℃ for up to one week.
Assay Procedure
Serum or Plasma Sample
1 drop of whole blood was added into sample pad, after all blood was completely absorbed. 1 drop of blood diluent was added and the result was observed in 10-20 minute.
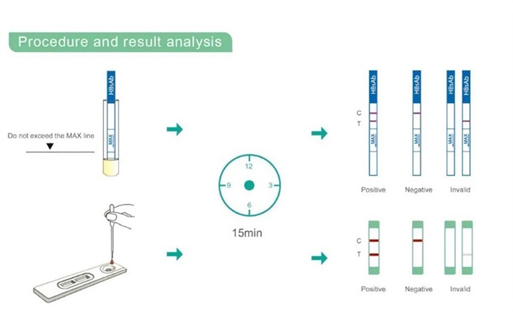
Procedure and result analysis
Interpretation Of Result
1. Negative: No apparent band in the test region (T), a pink-coloured band appears in the control region (C). This indicate that no HAV antibody has been detected.
2. Positive: In addition to a pink-coloured band in the control region (C), a pink colored band will appear in the test region (T). This indicates that the specimen contains HAV antibodies.
3. If no band appears in the control region(C), regardless of the presence or absence of line in the test region (T). It indicate a possible prior in performing the test. The test should be repeated using a new device.
Result
The provided data on HAV IgG and IgM antibody prevalence across different age groups, occupational groups (specifically health workers), educational levels, and marital statuses offers valuable insights into potential variations in exposure to Hepatitis A virus and immune responses.
The prevalence of IgG and IgM antibodies in the sample population was low with the overall prevalence of 3.2% for IgG and 2.4% for IgM .The highest prevalence of IgG antibodies was observed in the 19-29 age group, while the lowest prevalence of IgM antibodies was observed in women over 40.
The prevalence of IgG and IgM antibodies varied by occupation, health workers had the highest prevalence of both IgG and IgM antibodies at 2.4% for each. Students, artisans, and farmers had the lowest prevalence of IgG and IgM antibodies at 1.6% and 0.8% respectively.
The prevalence of IgG and IgM antibodies varied for educational level. Individuals with as a secondary education had the highest prevalence of IgG and IgM antibodies at 4.8% for each. Individuals with no education or a primary education had the lowest prevalence of IgG and IgM antibodies, at 1.6% and 0.8% respectively.
The prevalence of IgG and IgM antibodies varied by marital status. Married individuals had the highest prevalence of IgG and IgM antibodies, at 4.8% for each. Single, widowed, and divorced had the lowest prevalence of IgG and IgM antibodies at 0.8 % for each.
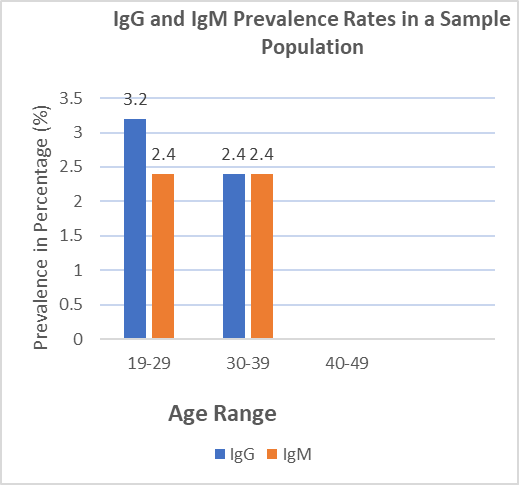
Fig 4.1: IgG and IgM Prevalence Rates in a Sample Population
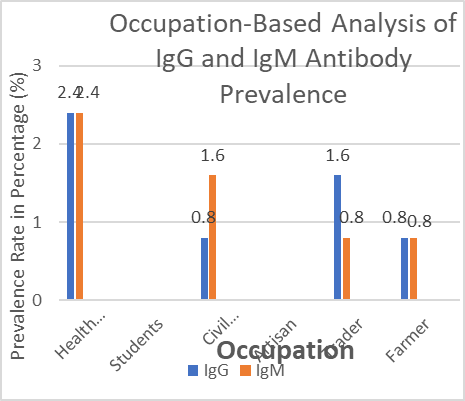
Fig 4.2: Occupation-Based Analysis of IgG and IgM Antibody Prevalence
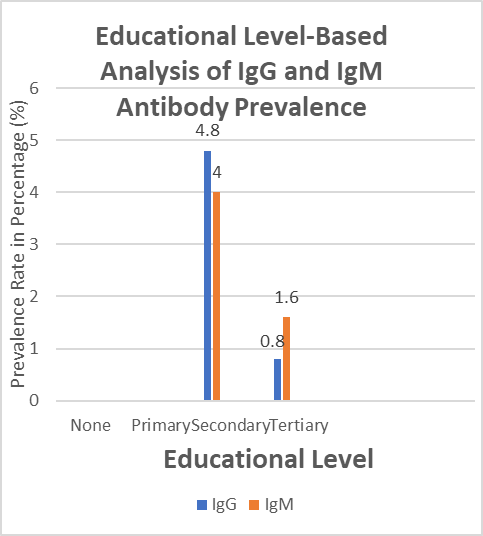
Fig 4.3: Educational Level-Based Analysis of IgG and IgM Antibody Prevalence
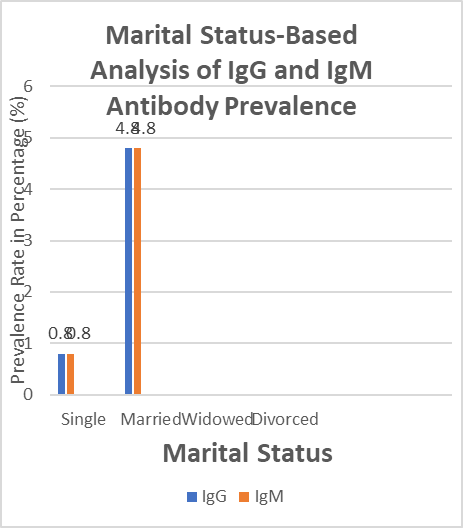
Fig. 4.4: Marital Status-Based Analysis of IgG and IgM Antibody Prevalence
Discussion
The study titled "Seroprevalence of Hepatitis A Virus Among Pregnant Women" looks into how common it is for pregnant women to have antibodies against the Hepatitis A Virus (HAV), specifically IgG and IgM. HAV-caused viral hepatitis is spread through the fecal-oral route, typically through direct person-to-person contact or the ingestion of contaminated food from an infected food handler. This can result in severe liver failure and death (Havelaar et al.,2015). Although it is difficult to determine the incidence during pregnancy, the purpose of this study is to evaluate the risk of acute infection and the amount of prior exposure to HAV (Rac et al., 2014). Understanding the HAV seroprevalence of pregnant women is crucial for providing educated prenatal care and developing public health policies, as they constitute a distinct population group with specific healthcare needs.
The data shows that the highest prevalence of HAV IgG and IgM antibodies is found in the 19–29 age range. This implies that there may be an increased risk of HAV infection for people in this age group. The population's overall prevalence of IgG (2.8%) and IgM (2.4%) antibodies shows a noticeable presence of both antibodies, with IgG being slightly more prevalent. These findings are consistent with those of other epidemiologic studies, such as those conducted in South Korea, where the prevalence of HAV infections among women increased from 151 cases in 2002 to 4,779 cases in 2009, with women aged 20–39 seeing the largest increase (Cho et al., 2013). This can be the result of a mix of past exposure and some persistent illnesses in the population.
Health workers are unique among the occupational groups under investigation, having the highest incidence of IgG and IgM antibodies.Given this increased prevalence, there may be a greater chance of HAV infection for healthcare professionals.They are probably more exposed to HAV at work because of things like touching sick patients or their bodily fluids.This idea is supported by the overall prevalence of IgG antibodies (1.6%) and IgM antibodies (1.2%), with IgG antibodies being slightly more common.This was further demonstrated by research results showing that health workers had a higher prevalence of hepatitis A virus antibodies than non-health workers. This was likely because health workers were more likely to be exposed to the virus through their jobs, such as through contact with infected patients or their bodily fluids. (Ojo et al., 2018).
Examining educational attainment reveals a notable disparity. People who have completed secondary school have much greater rates of IgG (4.8%) and IgM (4%) than people who have completed tertiary school (0.8% IgG and 1.6% IgM).This implies that differences in antibody prevalence according to educational attainment may exist, with a larger frequency being linked to secondary education. Nevertheless, additional investigation is required to comprehend the fundamental causes of this variation. According to data on marital status, the prevalence of both IgG and IgM antibodies is higher in married individuals (4.8% for both), but it is lower in single individuals (0.8% for both IgG and IgM). Sadly, there is no data available for divorced or bereaved people, which limits the conclusions that can be drawn about these groups. The results imply that variations in antibody prevalence may be related to marital status, with married people exhibiting a higher frequency than single people.
The results of this study have numerous significant connections to the results that have been previously presented. First, the age-based study shows that the largest incidence of HAV antibodies is seen in those between the ages of 19 and 29. Given that many of the women in this age range are of childbearing age, this demographic insight is consistent with the study's focus on pregnant women. Thus, it is critical to understand the frequency of HAV antibodies in pregnant women in this age group. It can have a big impact on prenatal treatment plans and how possible dangers related to HAV infection during pregnancy are managed.
Furthermore, the research of pregnant women has a great deal of relevance to the occupational risk factors that were previously described. Pregnant women may be exposed to certain occupational dangers, just like health workers are. This is particularly true if they work in hospital facilities or other places where there is a higher risk of HAV exposure. The results pertaining to health workers highlight the significance of determining the HAV seroprevalence in particular occupational groups. Depending on their occupations, pregnant women may potentially be at risk for HAV exposure.
awareness HAV seroprevalence requires an awareness of socioeconomic determinants. The preceding discussion's assessments of marital status and education levels provide important new perspectives on the ways in which socioeconomic variables may affect HAV seroprevalence. This information can be especially crucial for expectant mothers. It enables the customization of prenatal treatment, immunization plans, and medical protocols to address possible risk factors linked to HAV infection during pregnancy.
It is not only sensible but also vital to incorporate the research findings on HAV prevalence into the study of pregnant women. It offers a useful contextual framework for evaluating the possibility of contracting HAV infection during pregnancy as well as its possible consequences. Healthcare practitioners can use this information to tailor preventive interventions for expectant mothers, passive immune globulin prophylaxis, and prenatal care (Moro et al., 2014). Crucially, it draws attention to the particular requirements and hazards faced by this group in the larger framework of infectious disease prevalence and public health initiatives.
Conclusion
To sum up, the investigation on the seroprevalence of the Hepatitis A Virus (HAV) in expectant mothers closes a significant knowledge gap by offering crucial information about the frequency of HAV antibodies throughout pregnancy. The results highlight the necessity for specialized prenatal care and immunization programs, especially for women who are fertile or planning a family, in order to prevent HAV infection in both expecting mothers and their unborn children. The study expands the breadth of public health initiatives to address these inequities by taking into account occupational and socioeconomic determinants, which further enhances our understanding of how these variables can influence disease risk. Overall, this study adds to a more thorough understanding of the dynamics of infectious diseases in the pregnant population and has direct implications for enhancing the health of mothers and their offspring.
References
-
Ahmad, T., Adnan, F., Nadeem, M., (2018). Assessment of the risk for human health of enterovirus and hepatitis A virus in clinical and water sources from three metropolitan cities of Pakistan. Ann Agric Environ Med, 2(4), 708–13.
Publisher | Google Scholor -
Bizri, A. R., Fares, J., Musharrafieh, U. (2018). Infectious diseases in the era of refugees: hepatitis A outbreak in Lebanon. Avicenna J Med, 8(4), 147–52.
Publisher | Google Scholor -
Casey, L. C., Fontana, R. J., Aday, A., Nelson, D. B., Rule, J. A., Gottfried, M., Tran, M., & Lee, W. M. (2020). Acute liver failure (ALF) in pregnancy: how much is pregnancy-related? Hepatology.22(3):7-8
--> -
Indians and Alaska Natives, 1946-2005. Am J Epidemiol, 174(11 Suppl), S89–96.
Publisher | Google Scholor -
Chaudhry, S. A., and Koren, G. (2015). Hepatitis A infection during pregnancy. Can Fam Physician, 61(11), 963-4.
Publisher | Google Scholor -
Cho, G. J., (2013). Hepatitis A virus infection during pregnancy in Korea: hepatitis A infection on pregnant women. Obstetrics & Gynecology Science, 56, 368-374.
Publisher | Google Scholor -
Daudi, N., Shouval, D., Stein-Zamir, C., Ackerman, Z. (2012). Breastmilk hepatitis A virus RNA in nursing mothers with acute hepatitis A virus infection. Breastfeed Med, 7, 313–315.
Publisher | Google Scholor -
De Jong, G. (2007). Guidelines for the Control of Hepatitis A in South Africa. South Africa: National Institute of Communicable Disease.
Publisher | Google Scholor -
Di Cola, G., Fantilli, A. C., Pisano, M. B., & Ré, V. E. (2021). Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review. International Journal of Food Microbiology, 338, 108986.
Publisher | Google Scholor -
Elinav, E., Ben-Dov, I. Z., Shapira, Y. (2006). Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology, 130, 1129–1134.
Publisher | Google Scholor -
Foster, M., Ramachandran, S., Myatt, K., et al. (2018). Hepatitis A virus outbreaks associated with drug use and homelessness-California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep, 67(43), 1208–10.
Publisher | Google Scholor -
Havelaar, A. H., Kirk, M. D., Torgerson, P. R., et al. (2015). World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med, 12(12), e1001923.
--> -
Hettmann, A., Juhasz, G., Dencs, A., et al. (2017). Phylogenetic analysis of a transfusion-transmitted hepatitis A outbreak. Virus Genes, 53(1), 15–20.
Publisher | Google Scholor -
Jacobsen, K. H. (2018). Globalization and the changing epidemiology of hepatitis A virus. Cold Spring Harb Perspect Med, 8(10). 10.1101/cshperspect.a031716.
Publisher | Google Scholor -
Klevens, R. M., Miller, J. T., Iqbal, K., et al. (2010). The evolving epidemiology of hepatitis A in the United States: incidence and molecular epidemiology from population-based surveillance, 2005-2007. Arch Intern Med, 170(20), 1811–8.
Publisher | Google Scholor -
Moro, P. L., Museru, O. I., Niu, M., Lewis, P., & Broder, K. (2014). Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. American journal of obstetrics and gynecology, 210(6), 561-e1.
--> -
Ojo O.A.,Ogundele, O., et al.(2018).Occupational risk factors fpr hepatitis A virus infection among healthcare workers in Nigeria. BMC Public Health. (1):1003.
--> -
Ott, J. J., Irving, G., Wiersma, S. T. (2012). Long-term protective effects of hepatitis A vaccines. A systematic review. Vaccine, 31, 3–11.
Publisher | Google Scholor -
Purpari, G., Macaluso, G., Di Bella, S., (2019). Molecular characterization of human enteric viruses in food, water samples, and surface swabs in Sicily. Int J Infect Dis, 80, 66–72.
Publisher | Google Scholor -
Rac, M. W., Sheffield, J. S. (2014). Prevention and management of viral hepatitis in pregnancy. Obstet Gynecol Clin North Am, 41, 573–592.
Publisher | Google Scholor -
Schwarz, N. G., Revillion, M., Roque-Afonso, A. M., et al. (2008). A food-borne outbreak of hepatitis A virus (HAV) infection in a secondary school in Upper Normandy, France, in November 2006. Euro Surveill, 13(22), pii=18885.
Publisher | Google Scholor -
Tanaka, S., Kishi, T., Ishihara, A., et al. (2019). Outbreak of hepatitis A linked to European outbreaks among men who have sex with men in Osaka, Japan, from March to July 2018 Hepatol Res, 49(6), 705–10.
Publisher | Google Scholor -
Wang, X., (2015). Hepatitis A virus and the origins of picornaviruses. Nature, 517, 85-88
Publisher | Google Scholor -
Zeng, D.-Y., Li, J.-M., Lin, S., Dong, X., You, J., Xing, Q.-Q., Ren, Y.-D., Chen, W.-M., Cai, Y.-Y., Fang, K., & Zhang, L. (2021). Global Burden of Acute Viral Hepatitis and Its Association with Socioeconomic Development Status, 1990-2019. Journal of Hepatology. 10:1016
Publisher | Google Scholor
