REVIEW ARTICLE
- Dubale Beyene 1
Department of Veterinary Science, College of Agriculture and Natural Resources, Jinka University, Ethiopia
*Corresponding Author: Dubale Beyene, Department of Veterinary Science, College of Agriculture and Natural Resources, Jinka University, Ethiopia
Citation: Dubale Beyene, ANTIGEN-SPECIFIC ANTIBODIES APPLICATION ON TUBERCULOSIS, Biomedical Studies and Clinical Evaluations, vol 1(3). DOI: https://doi.org/10.64347/3064-7037/BSCE.012
Copyright: © 2024, Dubale Beyene, this is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: September 08, 2024 | Accepted: October 28, 2024 | Published: October 21, 2024
Abstract
Tuberculosis (TB) is caused by Mycobacterium tuberculosis (Mtb). Mtb is an airborne transmitted pathogen, and the immune responses, especially the mucosal immune response, play fundamental roles for the host to defend the primary and the containment of Mtb infection. It remains a significant health threat to mankind and is undoubtedly the most successful disease caused by a single infectious agent ever. TB killed ∼1.5 million individuals in 2018 alone, and a total of around 1,000,000,000 people over the last 200 years. The Bacillus Calmette‑Guerin immunization is the as it was authorized immunization against TB, but its protective impact does not amplify to controlling the improvement of irresistible pulmonary disease in grown‑ups. To develop improved vaccines and a new method for controlling TB, an important element is the discovery of markers to measure the effectors’ mechanisms of the protective immune response against M. tuberculosis. Humoral responses are conspicuous during active TB illness and have indeed been hypothesized to contribute to immunopathology. In any case, there's proof to recommend that particular antibodies may restrain the dispersal of MTB, and possibly play a part in the avoidance of disease through mucosal resistance. Further, antibodies are now understood to confer protection against a run of intracellular pathogens by modulating immunity via means of Fc‑receptor mediated phagocytosis. The objective of the present study will be to review antigen-specific antibodies application in Tuberculosis and their potential utility as biomarkers and their functional contribution to Mtb control.
Keywords: antibodies, humoral immunity, tuberculosis
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), remains a significant health threat to mankind and is undoubtedly the most successful disease caused by a single infectious agent ever (Cardona, 2016). TB killed ∼1.5 million individuals in 2018 alone, and a total of around 1,000,000,000 people over the last 200 years (WHO, 2019; Paulson, 2013).
Approximately one-fourth to one-third of the world’s population is infected with Mtb, giving rise to an estimated
10 million new cases annually (WHO, 2019). Mtb infection
leads to a spectrum of infectious states ranging from various levels of asymptomatic states, collectively referred to as latent tuberculosis infection (LTBI) and a spectrum of active tuberculosis diseases (ATB), ranging from local to pulmonary to disseminating ATB (Kawahara et al., 2019; Demartino, 2019). About 5 10% of individuals with LTBI will progress to ATB during their lifetime; the remainder can contain the infection lifelong unless immunosuppressed, such as by co-infecting viruses [e.g., human immunodeficiency virus (HIV)] or iatrogenically (Cardona, 2016; Joosten et al., 2013).
It is assist evaluated that 2 billion individuals right now keep up an inactive infection with Mycobacterium tuberculosis the causative agent of TB, and are subsequently at the chance of creating active disease at a few points during their lives (Clemens Hermann and Carolyn G. King, 2021). Thus, the development of rapid and accurate new diagnostic methods is vital for the global control of TB. However, the diagnostic accuracy of existing tests is inadequate (Wallis et al., 2013).
The Bacillus Calmette Guerin (BCG) antibody was presented to avoid disease during the mid-20th century but, despite far-reaching scope, has fizzled (Ashley et al., 2016). This arm of the resistant framework has been explored
Abstract
Tuberculosis (TB) is caused by Mycobacterium tuberculosis (Mtb). Mtb is an airborne transmitted pathogen, and the immune responses, especially the mucosal immune response, play fundamental roles for the host to defend the primary and the containment of Mtb infection. It remains a significant health threat to mankind and is undoubtedly the most successful disease caused by a single infectious agent ever. TB killed ∼1.5 million individuals in 2018 alone, and a total of around 1,000,000,000 people over the last 200 years. The Bacillus Calmette‑Guerin immunization is the as it was authorized immunization against TB, but its protective impact does not amplify to controlling the improvement of irresistible pulmonary disease in grown‑ups. To develop improved vaccines and a new method for controlling TB, an important element is the discovery of markers to measure the effectors’ mechanisms of the protective immune response against M. tuberculosis. Humoral responses are conspicuous during active TB illness and have indeed been hypothesized to contribute to immunopathology. In any case, there's proof to recommend that particular antibodies may restrain the dispersal of MTB, and possibly play a part in the avoidance of disease through mucosal resistance. Further, antibodies are now understood to confer protection against a run of intracellular pathogens by modulating immunity via means of Fc‑receptor mediated phagocytosis. The objective of the present study will be to review antigen-specific antibodies application in Tuberculosis and their potential utility as biomarkers and their functional contribution to Mtb control.
Keywords: antibodies, humoral immunity, tuberculosis
Biomedical Studies and Clinical Evaluations Winsome Publishing LLC
@ 2024 Dubale Beyene 2
during normal infection with Mycobacterium tuberculosis in detail (Flynn, 2001; Nunes-Alves et al., 2014); and even though imperative, isn't fundamentally adequate to avoid the microbes.
As a facultative intracellular bacterium that resides primarily in lung alveolar macrophages, the vast majority of TB research efforts have traditionally focused on understanding cell-mediated immunity (CMI)(Cooper, 2009; Ottenhoff, 2012), By contrast, the role of B-cell and antibody-mediated immunity (AMI) in TB has remained understudied for decades. This was due to the historical dogma, established in the early twentieth century, that postulated that host defense against intracellular pathogens is mediated by CMI, whereas the response to extracellular pathogens is mediated by Abs produced from B-cells (Kawahara et al., 2019; Csadevall, 2018). The MVA85A is one such vaccine and was as of late tried in two points of interest adequacy trials (Tameris et al., 2013; Ndiaye et al., 2015).
As Mycobacterium tuberculosis may be a facultative intracellular pathogen, it has been hypothesized that antibodies either have no protective advantage or may indeed contribute to immunopathology inactive infection (Orme, 2014). Surmounting this presumed lack of functional antibodies in TB presents a significant challenge for the next generation of immunizations against TB, as antibody titer and specificity remain the overwhelming connections of vaccine-mediated immunity for numerous other illnesses (Plotkin, 2008). In this manner, the objective of this paper is to review antigen-specific antibodies application in Tuberculosis and their potential utility as biomarkers, and their functional contribution to Mtb control.
2. Tuberculosis
Mycobacterium tuberculosis infection in people produces a range of clinical and subclinical infections (Milla et al., 2019). This continuum can be freely gathered into active TB, subclinical TB, latent TB, inactive TB, and “Resisters.” People with active TB have distinguishable bacillary burden by culture or PCR and commonly have a positive interferon-γ (IFN-γ) releasing assay (IGRA) or tuberculin skin test (TST) with cough, weight loss, and fever (Milla et al., 2019).
Subclinical TB is characterized as an asymptomatic illness but with a misfortune of bacterial control which can be watched with aggravation as identified radiographically by positron emission tomography (PET) (Milla et al., 2019). At the other conclusion of the range, inactive TB is distinguished as tireless asymptomatic contamination, TST or IGRA positive with no transmission capacity (Achkar and Jenny-Avital, 2011).
Not at all like subclinical TB, do people with inactive disease not illustrate zones of PET eagerness reliable with active illness (Milla et al., 2019). At last, a subset of TST and IGRA negative people have been recognized called “Resisters” who show up to have non-canonical safe reactions to mycobacterium tuberculosis antigens within the setting of high levels of introduction (Simmons et al., 2018).
2.1. Life Cycle of Mycobacterium Tuberculosis.
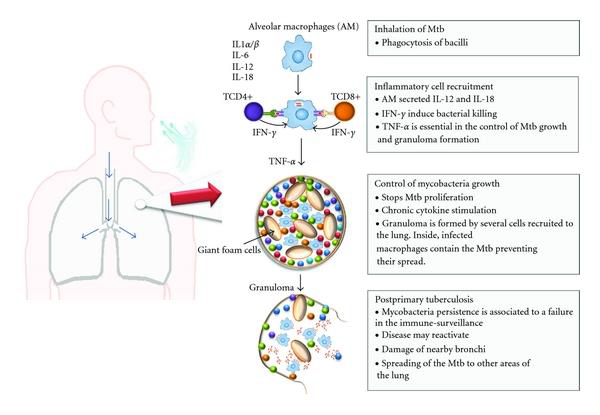
Person-to-person transmission occurs by inhalation of aerosolized droplets generated by a person with active disease. Bacteria travel to the lungs, where they are taken up by alveolar macrophages. Inside the alveolar macrophages, bacteria are exposed to reactive oxygen (ROS) and nitrogen species generated by macrophages. M. tuberculosis can evade macrophage killing by inhibiting phagosome-lysosome fusion. This leads to the recruitment of immune cells, which contributes to the formation of granulomas that can contain M. tuberculosis. In 90% of the cases, infected individuals contain the infection within the granuloma, where the bacteria can survive in a nonreplicating state, probably triggered by hypoxic and nutrient-starved conditions. In around 10% of cases, the disease will progress and develop into active disease, which can lead to the release of M. tuberculosis from the granulomas. In a small percentage of latently infected individuals, the disease can reactivate later in life leading to the development of active disease.
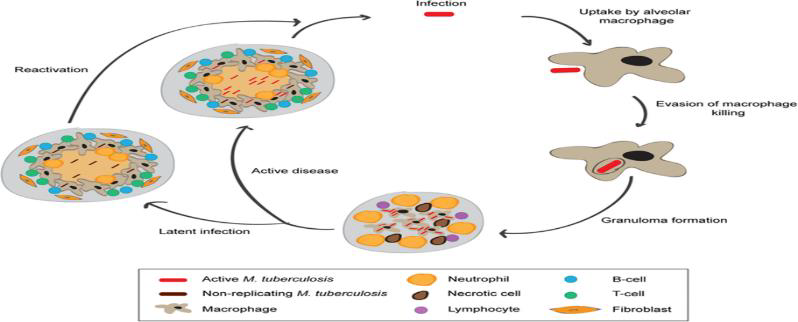
Figure 1: Schematic representation of the life cycle of M. tuberculosis
3. Humoral immunity during tuberculosis infection
3.1. B cells and Tuberculosis Antibody ResponsesBased on the concept of division of labor by the cell-mediated and the humoral arm of the immune response in controlling pathogens, protection against intracellular microbes is generally thought to be mediated exclusively by cell-mediated immunity (Casadevall, 2003). This has led to the use of highly T cell-centric strategies for the development of vaccines against intracellular pathogens including M. tuberculosis (Sender and Hill, 2000).Although antibodies are induced against a broad range of protein and non-protein antigens in active TB disease, they are not useful for diagnosis due to a lack of sensitivity and specificity. There is evidence for reduced antibody avidity in active TB disease and perturbation in Fc receptor expression, suggesting that phagocytosis and antibody-mediated cellular cytotoxicity could be dysregulated. Transcriptomic signatures for B cells are also depressed in TB, suggesting down-regulation or exhaustion of the B cell response (Scriba et al., 2017).
B cells and antibodies are involved in the immune response to TB, and the interaction of antibodies with phagocytic cells through Fc receptor engagement is emerging as a key area for research. The quantity of antibodies produced during M. tuberculosis infection is related to bacterial load, and higher antibody responses are observed in those at risk of disease and are correlated with the mycobacterial load during disease (Kunnath et al., 2012). This suggests that antibodies are important in the control of active TB disease and has also led to the development of antibody-based assays for TB diagnosis (Scriba et al., 2017).

Figure 3: How do B cells modulate the immune responses to M. tuberculosis?
Production of M. tuberculosis-specific antibodies can mediate the formation of an immune complex that can modulate the functions of effector cells such as dendritic cells and macrophages. It remains to be demonstrated whether specific neutralizing antibodies exist. B cells can serve as antigen-presenting cells to influence T cell activation, polarization, and effector functions and the establishment of T cell memory. B cells can also modulate the functions of granulomatous immune cells. In concert, these antibody-dependent and independent functions of B cells play an important role in determining disease outcome in terms of the elimination of control of bacteria, as well as the development of immunopathology that could damage tissues and promote dissemination (L. kozakiewicz et al., 2013).
3.2. Antibody Quality
The primary purpose of antibody measurement has been the diagnosis of TB disease, and most studies focus on the number of antibodies detected and not antibody quality. Antibody avidity is variable in TB patients (Maes RF, 1991), and shortly following TB treatment, there is an increase in antibody quantity and a decrease in avidity, suggesting exhaustion of the B cell response (Aris-Bouda LM et al., 2003). A decrease in avidity of antibody specific for the live cell surface of mycobacteria in TB patients, suggesting conversion of antibodies to low avidity IgG or B cell exhaustion (Perley et al., 2014). Antibody avidity was also higher in those previously immunized with BCG, which raises the possibility of using vaccination to improve antibody avidity as a potential mechanism for protection against TB (Scriba et al., 2017). 3.3. Variation in Human Antibody Responses against Mycobacterium Tuberculosis
It has long been known that natural infection induces the formation of antibodies against MTB (Jacobs et al., 2016). Studies demonstrated that 90% of TB patients have raised titers of serum immunoglobulin against mycobacterial antigens at the time of clinical presentation (Lyashchenko et al., 1998). The correlation between antibody responses and active TB disease led to an investigation of antibodies as diagnostic markers rather than a therapeutic strategy, but these efforts were discouraged by the WHO in 2012, due to suboptimal sensitivity and specificity in studies (WHO, 2021). 3.4. Isotypes and Subclasses of Antibodies to Mycobacterium Tuberculosis Infection Within the broadest of strokes, antibody titers increment as Mycobacterium tuberculosis burden increments, apparently due to expanded antigen accessibility (De- Araujo et al.,
Biomedical Studies and Clinical Evaluations Winsome Publishing LLC
@ 2024 Dubale Beyene 6
2018). More particularly, a few but not all think about appearing that dynamic aspiratory TB inspires Mycobacterium tuberculosis particular IgG isotype reaction with the rise of both Mycobacterium tuberculosis -particular and add up to IgG1 and IgG3 subclasses in serum (De- Araujo et al., 2018). In vitro, IgG1 mediates TNF-α discharge from human monocytes but does not increment the anti-inflammatory cytokine IL 10 (Hussain et al., 2001). Reliable with these affiliations, complement C1q levels are higher in dynamic TB compared to inactive TB, and complement cascade particles are up-regulated 18 months sometime recently movement from Mycobacterium tuberculosis infection to TB disease (Lubbers et al., 2018). In addition, a few monoclonal Mycobacterium tuberculosis IgG1 can upgrade bacterial replication in vitro, illustrating the potential to worsen illness (Zimmermann et al., 2016). Notably, the defensive impact shows up to be revoked with the enzymatic evacuation of glycan buildups (Olivares et al., 2013). Hence, Mycobacterium tuberculosis receptive IgG with intact glycosylation in complex polyclonal reactions may take an interest in controlling bacterial burden (Milla et al., 2019). Beyond IgG, IgA has been a center of interest due to the significance of this isotype in mucosal immunity inside the aspiratory compartment, the most courses of TB infection, acquisition, and ensuing transmission (Milla et al., 2019). Monoclonal IgA was able to repress Mycobacterium tuberculosis development, whereas IgG antibodies to the same targets advanced disease (Zimmermann et al., 2016). Intriguingly, this isotype-mediated control of MTB appears to be modulated via FcαR/FcγR independent mechanisms (Milla et al., 2019).
3.5. Antibodies as Potential Biomarkers for Protective Immunity against Mtb Individuals with persistent negative TSTs, despite years of exposure to ATB patients, had elevated anti-Mtb IgG levels, and their serum was able to block proliferation of peripheral blood mononuclear cells in response to protein purified derivatives (PPD) (171). In concordance, highly exposed, but TST-negative, individuals displayed high anti-PPD Abs titers, which inhibited autologous T-cell proliferation after PPD stimulation (172). Abs specific for CFP-10 and ESAT-6 in Quantiferon TB Gold (QFT) supernatants independently separated LTBI from ATB (173). More recently, Lu et al. reported that highly exposed, but TST- and IFN-γ release assay (IGRA)-negative, individuals harbored Mtb-specific IgM and IgG, while diminished CD4-mediated IFN-γ responses directed toward Mtb early secreted Ag of 6 kDa (ESAT-6), 10 kDa culture filtrate protein (CFP-10), Ag85A and Ag85B were found (163). Taken together, these studies implicate that humoral immunity is detectable infrequently exposed individuals with persistently negative skin testing or QFN evaluation, which represent read-outs of effector T-cell responses. In such settings, Abs may be considered biomarkers of protective immunity.
4. Antibody-mediated protection
4.1. Humoral Immunodeficiency and Risk of Tuberculosis
HIV is known to cause immune dysfunction, rendering HIV/TB-co-infected individuals more susceptible to progression to ATB (Esmail et al., 2018; Gupta et al., 2015). Specifically, progressive untreated HIV infection is associated with a loss of total (Esmail et al., 2018) and M. tuberculosis-specific CD4 T cells (Yao et al., 2014). Given the critical role of CD4 T cells in the control of TB in mice (Lin, 2012), depletion of T cells is likely to contribute to the development of ATB in HIV-infected individuals.
Genetic susceptibility to mycobacterial disease is well described and is typically seen in the loss of functionality in the IL-12, STAT1, and IFN-ℽ pathways (Cottle, 2011). IFN-γR1 deficiency resulting from homozygous or compound heterozygous null mutations in IFN-γR1 causes severe, often fatal infection (Newport et al., 1996; Jouanguy et al., 1997; Altare et al., 1998). A homozygous null mutation in IFN-γR2 also results in severe atypical mycobacterial infection (Dorman and Holland, 1998). In both deficiencies, granulomas fail to form in tissues. Both the binding (IFN-γR1) and signaling (IFN-γR2) chains of the IFN-γ receptor are essential for IFN-γ mediated signaling and control of mycobacterial infection. In partial IFN-γR1 deficiency, the IFN-γR1 is expressed on monocytes but has reduced ligand affinity (Jouanguy et al., 1997). In contrast, to complete IFN-γR1 deficiency, this reduced IFN-γ signaling is sufficient for the organization of granulomas, and the clinical course is milder.
4.2. Antibodies in Prevention of Infection with Mycobacterium Tuberculosis
Antibodies that have been obtained from persons with latent tuberculosis and those with active tuberculosis have functional differences in vitro. Antibodies from peripheral-blood cells of persons with the latent disease and active disease have the efficacy of inhibiting Mycobacterium tuberculosis infection in macrophages and epithelial cells (Lu et al., 2016 and Zimmermann et al., 2016). IgG from persons with the latent disease was more effective at inhibiting mycobacterial growth in macrophages than IgG from persons with active disease (Lu et al., 2016). IgA isotypes inhibited infection of an epithelial cell line and the IgG isotypes promoted infection (Zimmermann et al., 2016).
4.3. Monoclonal Antibody Studies during Mycobacterium Tuberculosis Infection in Mice
The defensive impact of monoclonal antibodies focuses on three well-known MTB antigens: heparin-binding hemagglutinin (HBHA), alpha-crystallin, and arabinomannan (AM), the sugar component of the glycolipid lipoarabinomannan (LAM) (Clemens, H, and Carolyn, G. King, 2021). Monoclonal antibodies decreased bacterial burden, improved control of microbes, or diminished lung pathology (Balu et al., 2021). HBHA is a surface-exposed protein that interacts with proteoglycans and can facilitate MTB entry into epithelial cells in vitro (Locht et al., 2006).
During infection, HBHA was shown to be required for extrapulmonary dissemination, as mucosal administration of MTB lacking HBHA expression impaired its ability to spread to other organs, such as the spleen (Pethe et al., 2001). Similar to HBHA, LAM is found within the bacterial cell envelope and constitutes a major component of the cell wall (Clemens, H, and Carolyn, G. K, 2021). Alpha-crystallin (too called 16-kDa antigen or HspX) may be a cytosolic protein that has moreover been recognized within the cell envelope of MTB (Hermann et al., 2021). Accordingly, alpha-crystallin is essential for bacterial survival during periods of disease latency when MTB also undergoes metabolic adaptation to survive under conditions of oxygen deprivation, nutrient depletion, and low pH (O’Toole et al., 2018).
4.4. Potential Mechanisms of Antibody-Mediated Immunity in Tuberculosis
Despite being a facultative intracellular pathogen, MTB is potentially susceptible to various mechanisms of antibody-mediated immunity. Opsonization through FcγR was shown to promote phagolysosomal fusion (Armstrong and Hart, 1975) and to increase macrophage Ca2+ signaling and intracellular killing (Malik et al., 2000). IgG bound to BCG increased the release of oxygen in the phagosomes of alveolar macrophages, suggesting the enhancement of antimycobacterial macrophage activity by antibody (Suga et al., 1996). Immune complexes that stimulate
FcεRII CD23 receptors trigger has been associated with antimycobacterial activity (Mossalayi et al., 2009).
Furthermore, the existence of potentially synergistic functions between humoral and cell-mediated immunity against TB is suggested by the observation that anti-mycobacterial antibodies in BCG-vaccinated persons enhance both innate and cell-mediated immune responses against mycobacteria (de Vallière et al., 2005) and that sera from TB contacts with high but not low IgG titers against tuberculin can block proliferation of PBMC cultures with tuberculin (Encinales et al., 2010). Moreover, a robust T cell response against mycobacteria is enhanced by specific antibody responses that can augment Th1 activation via FcR by facilitating rapid uptake, processing, and presentation of antigens (Igietseme et al., 2004).
The antibody can also contribute to the host defense against MTB by promoting the clearance of immunomodulatory antigens such as LAM (Glatman-Freedman et al., 2000). Finally, antibodies that mimic the action of fungal killer toxins are bactericidal to M.tb (Conti et al., 1998). Although such antibodies are unlikely to be present in MTB infection, the fact that mycobacteria can be killed directly by certain antibodies provides a precedent for such a mechanism of antibody-mediated protection.
In addition to these direct mechanisms, antibodies can influence the outcome of mycobacterial infection through their ability to modulate inflammation. Some antibodies, such as IgM, can demonstrate pro-inflammatory properties through their ability to activate complement (Ciurana et al., 2004), while other antibodies, such as IgG, can demonstrate pro- or anti-inflammatory properties depending on the antigen and FcR receptor engaged (Ballow, 2011; Lux et al., 2010).
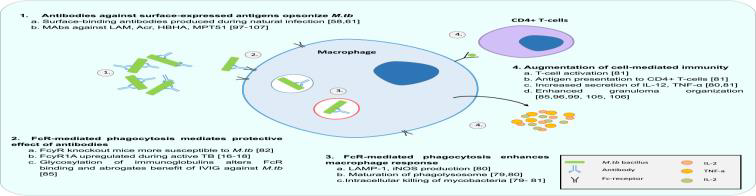
Figure 4: Antibodies modulate MTB-macrophage interaction via FcR mediated phagocytosis
Antibodies are now also understood to augment CMI and reduce the survival of intracellular pathogens via effector functions of the Fcγ receptor (FcR) (Tameris et al., 2013).
4.6. Mucosal Protection against Tuberculosis
It is well known that the mucosa is the largest immune organ in the body, and it is generally believed that almost all infectious diseases are initiated at the mucosal surface (Ye et al., 2011). The respiratory tract is the natural route for Mtb infection, where Mtb infects the individual mainly through the mucosal tissue of the respiratory tract after inhalation of mycobacteria containing droplets from the external environment. Normally, the pathogen (Mtb) infection could be eliminated by the host’s immune system, but it is desirable to induce immunity before the infection through vaccination in most cases. To effectively prevent Mtb infection, the approach of mucosal immunization has recently received increasing attention in the field of tuberculosis vaccination owing to its potency in inducing mucosa-associated protection from mucosal infectious diseases (Ogra et al., 2001). Several lines of evidence have suggested that mucosal immunity can provide unique advantages for protection against mycobacterial infection, by which the immune cells, such as macrophages, dendritic cells, and leukocytes recognize the pathogen-associated molecular patterns (PAMPs), and sequentially activate the anti-mycobacterial immune responses including the activation of specific T-cell and antibody synthesis (Mogensen, 2009).
Mice missing the polymeric Ig receptor which transports IgA into the respiratory mucosa are more helpless to MTB infection than wild-type mice (Tjarnlund et al., 2006). In a novel try, polyclonal human secretory IgA (hsIgA) was decontaminated from colostrum given by healthy women and appeared to contain IgA able to tie entirety BCG and MTB lysate (Alvarez et al., 2013).
Prophylactic intra-tracheal incubation or pre-incubation of MTB with this hsIgA diminished bacillary stack and moved forward granuloma arrangement within the lungs of mice challenged with live MTB (Alvarez et al., 2013). This appeared that antibodies able of interacting with MTB within the mucosa may be passively passed on between mother and child, which human MB particular hsIgA can alter the course of disease (Alvarez et al., 2013).
4.7. Antibody Function in Mycobacterium tuberculosis
With differential glycosylation, isotypes, and subclasses, antibody Fc-domain engagement of Fc receptors on immune cells helps modulate pro- and anti-inflammatory signals, the balance of which, in TB, can contribute to outcome (Hogarth, 2012). Despite lower Mycobacterium tuberculosis particularly IgG titers in inactive compared to active TB, there is a higher affinity for the activating FcγRIIIa and no difference in the activating FcγRIIa or inhibitory FcγRIIb (Lu et al., 2016).
Studies in mice deficient of the inhibitory FcγRIIb have greater control of MTB bacterial burden, whereas complete knock out of the Fcγ chain region, which is essential for activating FcγR signaling, resulted in more severe disease in pulmonary pathology (Maglione et al., 2008). Furthermore, there are higher levels of FcγRI receptor quality expression in people with active TB compared to inactive TB (Sutherland et al., 2014), and these levels diminish throughout therapy (Cliff et al., 2013). FcγRI is an activating receptor, which is upregulated by cytokines such as IFN-γ and GM-CSF and binds with high affinity to IgG1, IgG3, and IgG4 in comparison to the low-affinity FcγRIIIa or IIa (Hogarth, 2012).
As such, high FcγRI levels in active TB may either be a marker of or contribute to the inflammation. At a cellular level, immune cells expressing Fc receptors have been implicated in both the enhancement of bacterial uptake, as well as the control of bacterial fate, in the context of antibodies (Milla et al., 2019). In studies with human cells and Mycobacterium tuberculosis, macrophages, NK cells, and alveolar epithelial cells have been illustrated to both possibly repress additionally upgrade bacterial development within the setting of antibodies (Milla et al., 2019).
Be that as it may, upon the expansion of filtered polyclonal IgG after Mycobacterium tuberculosis disease, the intracellular bacterial burden is diminished within the setting of antibodies from inactive compared to active TB people (Lu et al., 2016). Particularly, the classical antibody-inspired effector capacities of NK cell actuation and consequent generation of granulysin to interceded ADCC have been watched (Lu et al., 2016; Roy et al., 2018).
Additionally, phagolysosomal fusion, inflammasome activation, and IL-1β production could be elicited more with polyclonal IgG purified from individuals with latent compared to active TB (Lu et al., 2016). Typically steady with monoclonal antibodies upgrading BCG lysosomal colocalization (Joller et al., 2010). Pre-hatching with human serum containing mycobacterial particular IgG and IgM encourage upgrades to complement authoritative to mycobacteria (Carrol et al., 2009).
4.8. The Antibody Reaction after Immunization
Mucosal or intravenous BCG inoculation in macaques was too as of late appeared to actuate close sterilizing resistance to MTB infection challenge, connecting with an increment in MTB-specific IgG, IgA, and IgM antibodies within the blood and bronchoalveolar lavage liquid (BAL) liquid (Dijkman et al., 2021).
IgG and IgM antibodies separated from the BAL of BCG immunization macaques have in this way been appeared to opsonize MTB and upgrade bacterial take-up by macrophages in vitro (Dijkman et al., 2021). Notably, MTBVAC—a live weakened MTB inferred immunization that evokes more vigorous and fast versatile resistant reactions compared with BCG (Dijkman et al., 2021).
Interestingly, oral administration of BCG led to increased anti-LAM IgA titers in tears, underscoring its capacity to induce mucosal antibody responses (Brown et al., 2003). Another ponder on intradermal BCG inoculation of members detailed increased AM-specific IgG reactions within the serum, which correlated with opsonization and killing of BCG by human macrophages (Chen et al., 2016).
Here, elevated Ag85A-specific IgG within the serum is related to diminished hazard of illness after BCG inoculation (Fletcher et al, 2016). At long last, and comparative to MTB disease, BCG immunization was appeared to initiate high avidity IgG antibodies to bulk surface antigens, like tuberculosis glycolipid (Nabeshima et al., 2015).
4.9. Antigens Targeted by Humoral Immunity during Tuberculosis Infection
Nevertheless, the foremost regularly recognized antigens for serological think about incorporating alpha-crystallin, HBHA, AM/LAM, antigen 85 (Ag85), PstS1, LpqH, MPT32, and malate synthase G (Steingart et al., 2009). Most of these antigens are recognized within the cell envelope and are at the slightest somewhat included in bacterial interaction with macrophages. Most of these antigens are at the slightest incompletely found within the
Biomedical Studies and Clinical Evaluations Winsome Publishing LLC
@ 2024 Dubale Beyene 9
cell envelope of MTB, highlighting the significance of this layer in creating antibody reactions amid disease (Clemens Hermann and Carolyn G. King, 2021).
The Ag85 complex, consisting of the subunits Ag85A, Ag85B, and Ag85C, is a secreted protein that also exhibits cell wall glycosyltransferase activity and is required for the biosynthesis of cord factor, a virulent glycolipid that drives granuloma formation (Belisle et al., 1997). Ag85 has a high affinity for fibronectin and facilitates the attachment of MTB to murine alveolar macrophages. PstS1 is a surface-exposed lipoprotein involved in the uptake of inorganic phosphate, an essential but often limiting nutrient in the microenvironment. Like Ag85, PstS1 can also act as an adhesin for binding to human and mouse macrophages (Esparza et al., 2015).
LpqH, another cell surface-exposed antigen, is a glycoprotein that acts as a TLR2 agonist, inducing up-regulation of MHC II and cytokine secretion by macrophages (Greenway et al., 2005; Nossa et al., 2001). The antigen MPT32 is secreted by MTB early on during disease progression but has also been detected in the cell envelope fraction of MTB. MPT32 functions as an adhesin and is suggested to be involved in the invasion of epithelial cells (Abeba et al., 2007). Lastly, malate synthase G is a cytosolic protein that functions in glycolate metabolism and elicits strong antibody responses during active TB (Laal et al., 1997).
Most of these antigens are at least partly located in the cell envelope of MTB, highlighting the importance of this layer in generating antibody responses during infection. In general, antibodies that bind to cell-surface exposed antigens can lead to opsonization, thereby impacting bacterial uptake and intracellular trafficking by phagocytic cells (Clemens Hermann and Carolyn G. King, 2021).
4.10. Attempts to Connect Antibody Specificity and Tuberculosis Illness
MTB disease is clinically tested by two tests: the tuberculin skin test (TST) and the interferon-ℽ (IFN-ℽ) release assay (IGRA). The TST involves an injection of PPD into the skin, which results in a delayed-type hypersensitivity reaction in individuals with previous MTB exposure or infection. IGRA measures IFN-ℽ production by MTB-specific T cells following ex vivo stimulation with MTB peptides, ESAT6, and CFP10 (Clemens Hermann and Carolyn G. King, 2021).
Individuals who stay TST and IGRA despite high presentation to individuals with clinically analyzed MTB have been named ‘resisters’ or ‘long-term controllers’ (Simmons et al., 2018). Antibodies from the serum of resisters were responsive to PPD, Ag85, ESAT6/CFP10, alpha-crystallin, GroES, and LAM (Clemens Hermann and Carolyn G. King, 2021). Although the specificities of antibodies isolated from resisters and individuals with latent TB infection were largely overlapping, bulk PPD-specific antibody responses in resisters were qualitatively distinct from individuals with latent TB, with higher avidity IgG and higher titers of antibodies capable of eliciting IFN-ℽ secretion of NK cells (Lu et al., 2019).
Although glycosylation of the Fc antibody domain regulates IgG structure and effector function, the link between glycosylation and function reflects changes in antibody specificity. Increased galactosylation that correlates with reduced antibody efficacy during active TB could represent the expansion of nonspecific plasma cells, which poorly target MTB (Clemens Hermann and Carolyn G. King, 2021).
MTB-specific plasma blasts appeared a high recurrence of IgA+ B cells proposing their mucosal root. Importantly, IgA antibodies were able to prevent epithelial cell infection in vitro while IgG antibodies with identical specificity either promoted infection or had no effect (Clemens Hermann and Carolyn G. King, 2021). In MTB infection, the high plenitude of IgG and IgA plasma cells within the lung is connected with a high bacterial load (Gideon et al., 2020). IgG antibodies focusing on AM in latently infected patients were found to upgrade take-up of MTB and intracellular killing by human macrophages in vitro. This impact was subordinate to anti-AM-specific antibodies since consumption of AM particular antibodies repealed the impact (Chen et al., 2020).
5.Potential future directions for antibody research in tuberculosis
Future applications of antibody formulations for the control of TB may include several possibilities including treatment, prevention, and diagnosis.
5.1. Treatment
Antibody-based therapy could potentially be useful in several scenarios. They could be used to shorten the standard treatment period of patients with uncomplicated TB when coupled with standard chemotherapy. However, they would be particularly important in the treatment of patients infected with Multi-drug Resistant (MDR) and Extensively Drug-Resistant (XDR) strains, in combination with the standard treatment (Armando et al., 2017).
Monoclonal antibodies against the surface of Methicillin-resistant Staphylococcus Aureus (MRSA) have very recently been used to deliver antibiotics directly to host cells, where MRSA appears to establish intracellular reservoirs to evade host immunity (Lehar et al., 2015). Utilizing comparable novel antibody-antibiotic conjugate innovation, Mabs seem possibly to be adjusted to provide drugs straightforwardly to TB-infected have cells (Ashley et al., 2016).5.2. Prophylactic use
Biomedical Studies and Clinical Evaluations Winsome Publishing LLC
@ 2024 Dubale Beyene 10
Prophylactic use of antibodies could be applied in recent contacts of TB patients, with special attention to risk groups (Norazmi et al., 2005). In this regard, successful prophylactic use of antibodies in exposed individuals has been shown in the case of several other pathogens such as varicella, tetanus, Respiratory Syncytial Virus (RSV), rabies, and Hepatitis B (Casadevall et al., 2004).
5.3. Vaccines
The induction of specific protective antibody responses by vaccination, either alone or as an addition to the stimulation of cell-mediated immunity could be a novel strategy for the development of the new generation of prophylactic and therapeutic vaccines against TB.
The prevailing past dogma that discounted the role of antibodies in host protection against TB has resulted in a limited study of B cell immunodominant epitopes as targets for protective immunity (DeGroot et al., 2005).
5.3.1. Polysaccharide conjugate vaccines
Polysaccharide conjugate vaccines are considered to elicit specific protective antibody responses against a variety of pathogens (Pollard et al., 2009). However, the polysaccharide conjugate vaccine against Salmonella typhi (Thiem et al., 2011) demonstrates the feasibility of this kind of vaccine for the prevention of infectious diseases caused by intracellular pathogens. In the case of M. tuberculosis, several authors reported the use of polysaccharide conjugated vaccine candidates (Glatman Freedman et al., 2004). Arabinomannan protein conjugate immunization initiated both antibody and T-cell reactions. Counteraction of LAM-induced T-cell suppression and inhibition of macrophage function by an antibody is another potential mechanism (Hamasur et al, 2003).
All these vaccine candidates induced the production of specific IgG (Schwebach et al., 2002) and some of them conferred variable levels of protection (Hamasur et al., 2003) which validate this strategy as one of the potential avenues for the development of a new generation of vaccines against tuberculosis.
5.3.2. Identifying other B-cell immunodominant epitopes
With the development of bioinformatics tools for bacterial genome analysis, it has been possible to predict in silico microbial regions that trigger immune responses relevant for protection and vaccine development. A candidate experimental vaccine based on proteoliposomes from M. smegmatis is currently in development (Rodriguez et al., 2011). In one study, a bibliographic search was used to identify highly expressed proteins inactive, latent, and reactivation phases of TB (LeThuy et al., 2010). The subcellular localization of the selected proteins was defined according to the report on the identification and localization of 1044 M. tuberculosis proteins using two-dimensional, capillary high-performance liquid chromatography coupled with mass spectrometry (2DLC/MS) method (Mawuenyega et al., 2005) and using prediction algorithms of a new generation of vaccines against tuberculosis.
In addition to cellular immune effectors recognizing antigens from M. tuberculosis, cross-reactive humoral immune responses of several IgG subclasses corresponding with a combined Th1 and Th2 pattern against antigenic components of M. tuberculosis were elicited. These findings were in concordance with the in silico predictions (LeThuy et al., 2010). It is interesting to note that differences in the pattern of humoral recognition of lipidic components were dependent on the characteristics of the adjuvant used, which could have relevance for the development of vaccines that includes lipidic components (Rodriguez et al., 2011).
Bioinformatics tools for the prediction of T and B epitopes were also employed for the design of multi-epitopic constructions, which were used to obtain recombinant BCG strains. Based on this prediction, B cell epitopes from ESAT-6, CFP-10, Ag87B, and MTP40 proteins were selected and combined with T cell epitopes of the 87B protein and fused to Mtb8.4 protein (Acosta et al., 2010).Next-generation sequencing has moreover contributed to the illustration of the Antibodies collection in reaction to infection and immunization (Georgiou et al., 2014). This progress may permit mapping of how the antibody reaction creates amid active TB infection, and how it is subverted by MTB to avoid the arrangement of any defensive antibodies as proposed by the antigenic variety in B-cell epitopes and the need for surface-binding antibodies (Ashley et al., 2016). The thinks about clinical populaces who show up to be safe to securing inactive disease with MTB are too of extraordinary intrigued in arrange to get it resistance against TB (Fletcher et al., 2016).
5.3.3. Diagnosis
Although no serological assays are currently recommended for the diagnosis of TB (Morris, 2011), largely due to the possibility of false results and thus incorrect treatments, for many other pathogens, serological diagnostic tests have been of great value, particularly in poor countries. In some cases, antibody responses can constitute useful correlates of protection (Edwards, 2001). In the specific case of TB, several studies of the antibody response have been reported (Velayudhan and Gennaro, 2010).
There is a substantial amount of variability in antibody response to TB (Navon et al., 2003). This variability has been attributed to several factors. Some of these factors are associated with the pathogen (strain variation, microenvironment, and growth state of bacteria) and others are related to the host, primarily previous exposure to related antigens and host genetics [99]. However, it is important to consider that only a small fraction of the genomic regions of M. tuberculosis encoding proteins have
Biomedical Studies and Clinical Evaluations Winsome Publishing LLC
@ 2024 Dubale Beyene 11
been explored. Currently, novel immunoassay platforms are being used to dissect the entire proteome of M. tuberculosis, including reacting protein microarrays with sera from TB patients and controls [101,102]. These studies could lead to the discovery of new antigens that may constitute suitable diagnostic markers and tools for the identification of protection correlates.
6. Conclusion and recommendations
Infection and illness caused by MTB emphatically invigorate humoral resistance in people. Even though CMI remains the overwhelming relate of security, there's proof to recommend that antibodies may contribute, at the slightest in portion, to resistance. The nearness of antibodies against particular MTB antigens such as LAM shows up to vary in patients with pneumonic and dispersed TB.
This compares to mAbs against LAM and HBHA that can diminish the bacillary stack and avoid dispersal of mycobacterial disease. Antibodies are presently broadly caught on to tweak CMI through FcR official and especially surface-binding antibodies in cases of mycobacterial contamination in test models. It is hence of intrigued that such antibodies don't appear to be invigorated amid normal disease, and the trend in protective mAbs hence distant could be a proposal that surface official with focusing on to FcR for improvement of CMI can happen in vivo.
These discoveries point to the require for assist testing of whether antibodies may bestow predominant security in immunization by improving CMI, or can avoid contamination on the off chance that shows earlier to have experience with MTB. Numerous challenges stand in this way, such as a need for information concerning the presence of useful mAbs in people and which epitopes are likely to initiate their arrangement. Be that as it may, modern innovations presently exist to conclusively address the achievability of joining objectives to target AMI within the plan of the following generation of antibody candidates.
There's a recommendation that Antibody-based therapy could potentially be useful to shorten the standard treatment period of patients with uncomplicated TB when coupled with standard chemotherapy. The study of the antibodies application in tuberculosis opens new possibilities for future development of new vaccines, diagnostics tools, and therapies against mycobacterium tuberculosis. Discoveries will likely arise from the ongoing studies in this area that will expedite the introduction of new strategies in the fight against tuberculosis.
Conflicts of Interest
The author declare no conflicts of interest.
References
-
A, Mehra., A, Zahra., V, Thompson., N, Sirisaengtaksin., A, Wells., M, Porto., S, Köster., K, Penberthy., Y, Kubota., A, Dricot. (2013): Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog, 9, Pp. e1003734.
Publisher | Google Scholor -
Glatman-Freedman, A.J, Mednick, N., Lendvai, A, Casadevall. (2000): Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infect. Immun, 68, Pp. 335-341.
Publisher | Google Scholor -
Abebe, F., Holm Hansen, C., Wiker, HG. (2007): Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand J Immunol, 66:176–91.
Publisher | Google Scholor -
Achkar, J. M., JennyAvital, ER. (2011): Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J Infect Dis, 204 (Suppl 4): S1179–86. DOI: 10.1093/indices/jir451.
Publisher | Google Scholor -
Acosta A, Norazmi MN, Sarmiento ME. (2010): Antibody-mediated immunity- a missed opportunity in the fight against tuberculosis? Malaysian J Med Sci, 17(2):68-7.
Publisher | Google Scholor -
Altare, F., Jouanguy, E., Lamhamedi Cherradi, S. (1998): A causative relationship between mutant IFNgR1 alleles and impaired cellular response to IFN gamma in a compound heterozygous child. Am. J. Hum. Genet, 62: 723–6.
Publisher | Google Scholor -
Alvarez, N., Otero, O., Camacho, F., Borrero, R., Tirado, Y., et al. (2013): A passive administration of purified secretory IgA from human colostrum induces protection against Mycobacterium tuberculosis in a murine model of progressive pulmonary infection. BMC Immunol, 14 (Suppl. 1):S3.
Publisher | Google Scholor -
Andrea, M. Cooper (2009): Cell-mediated immune responses in Tuberculosis. Annu Rev Immunol, 27: 393–422.
Publisher | Google Scholor -
Arias-Bouda, LM. Kuijper, S., Van der Werf, A., Nguyen, LN., Jansen, HM. Kolk, AH. (2003): Changes in acidity and level of immunoglobulin G antibodies to Mycobacterium tuberculosis in sera of patients undergoing treatment for pulmonary tuberculosis. Clin Diagn Lab Immunol, 10:702-709.
Publisher | Google Scholor -
Armstrongng, P.D., and Hart (1975): Phagosome lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usuanonfusionon pattern and observations on bacterial survival. J. Exp. Med, 142, Pp. 1-16.
Publisher | Google Scholor -
Ashenafi, S., Aderaye, G., Bekele, A., Zewdie, M., Aseffa, G., et al. (2014): Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin Immunol, 151:84-99.
Publisher | Google Scholor -
Ashley, J., Jacobs, Juthathip, Mongkolsapaya., Gavin, R., Screaton, Helen., McShan, Robert, J., Wilkinson. (2016): Antibodies and tuberculosis. Tuberculosis, 101: 102-113.
Publisher | Google Scholor -
Ballow (2011): The IgG molecule as biological immune response modifier mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J. Allergy Clin Immunol, 127, Pp. 315-323.
Publisher | Google Scholor -
Belisle, JT., Vissa, VD, Sievert, T. (1997): Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science, 276:1420–22.
Publisher | Google Scholor -
C.J. Cambier., S. Falkow., L. Ramakrishnan. (2014): Host evasion and exploitation sches of Mycobacterium tuberculosis Cell, 159, Pp. 1497-1509.
Publisher | Google Scholor -
C.L. Ciurana., B. Zwart., and G. van Mierlo., C.E. (2004): Hack Complement activation by necrotic cells in normal plasma environment compares to that by late apoptotic cells and involves predominantly IgM. Eur J Immunol, 34, Pp. 2609-2619.
Publisher | Google Scholor -
Cai, Y., Yang, Q., Tang, Y., Zhang, M., Liu, H., Zhang, G., Deng, Q., Huang, J., Gao, Z., Zhou, B., Feng, CG, Chen, X. (2014): Increased complement C1q level marks active disease in human tuberculosis. PLoS One, 9: 923-40.
Publisher | Google Scholor -
Cardona P. (2016): The progress of therapeutic vaccination about tuberculosis. Front Microbiol, 7:1536. doi: 10.33DOIfmicb.2016.01536
Publisher | Google Scholor -
Carroll, MV, Lack, N., Sim, E., Krarup, A., Sim, RB. (2009): Multiple routes of complement activation by Mycobacterium Bovis BCG. Mol Immunol, 46:3367-3378.
Publisher | Google Scholor -
Casadevall, A. (2018): Antibody-based vaccine strategies against intracellular pathogens. Curr Opin Immunol, 53:74–80. DOI: 10.1016/j.coi.2018.04.011.
Publisher | Google Scholor -
Casadevall, A. (2003): Antibody-mediated immunity against intracellular pathogens: two-dimensional thinking comes full circle. Infect Immun, 71(8):4225–4228.
Publisher | Google Scholor -
Casadevall, A., Dadachova, E., Pirofski, LA. (2004): Passive antibody therapy for infectious diseases. Nat Rev Microbiol, 2(9):717-723.
Publisher | Google Scholor -
Chackerian, A., Alt, J., Perera, T., Dascher, C., SMB. (2002): Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun, 70:4501–9. PubMed: 12117962.
Publisher | Google Scholor -
Chen, T., Blanc, C., Liu, Y. (2020): Capsular glycan recognition provides antibody-mediated immunity against tuberculosis. J Clin Invest, 130: 1808–22.
Publisher | Google Scholor -
Chen, T., Blanc, C., Eder, AZ. (2016): Association of human Abs to arabinomannan with enhanced mycobacterial opsonophagocytosis and intracellular growth reduction. J Infect Dis, 214:300–10.
Publisher | Google Scholor -
Clemens, H., Carolyn, G., King. (2021): TB or not to be: what specificities and impact do antibodies have during tuberculosis? Oxford Open Immunology, 2(1): iqab015.
Publisher | Google Scholor -
Cliff, JM., Lee, J-S., Constantinou, N., Cho, J-E., Clark, TG., et al. (2013): Distinct phases of blood gene expression pattern through tuberculosis treatment reflect modulation of the humoral immune response. J Infect Dis, 207:18-29.
Publisher | Google Scholor -
Colditz, G., Brewer, T., Berkey, C., Wilson, M., Burdick E. (1994): Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA, 271:698–702. [PubMed: 8309034].
Publisher | Google Scholor -
Conti, S., F, Fanti, W, Magliani, M., Gerloni, D., Bertolotti, A., Salati, A., Cassone, L, Polonelli (1998): Mycobactericidal activity of human natural, monoclonal, and recombinant yeast killer toxin-like antibodies. J. Infect. Di, 177, Pp. 807-811.
Publisher | Google Scholor -
Cooper, AM. (2009): Cell-mediated immune responses in tuberculosis. Annu Rev Immunol, 27:393–422.
Publisher | Google Scholor -
Cottle, LE. (2011): Mendelian susceptibility to mycobacterial disease. Clin Genet, 79:17-22.
Publisher | Google Scholor -
De Valliere, S., Abate, G., Blazevic, A., Heuertz, RM., Hoft, DF. (2005): Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun, 73: 6711-20.
Publisher | Google Scholor -
De Araujo, LS., Da Silva, NBM., Leung, JAM., Mello, FCQ., Saad, MHF. (2018): IgG subclasses’ response to a set of mycobacterial antigens in different stages of Mycobacterium tuberculosis infection. Tuberculosis,
Publisher | Google Scholor -
De Groot, AS., McMurry, J., Marcon, L. (2005): Developing an epitope driven tuberculosis (TB) vaccine. Vaccine, 23(17-18):2121-31.
Publisher | Google Scholor -
De Martino, M., Lodi, L., Galli, L., Chiappini, E. (2019): Immune response to Mycobacterium tuberculosis: a narrative review. Front Pediatr, 7: 350.DOI: 10.3389/fped.2019.00350.
Publisher | Google Scholor -
Demangel C, Bertolino P, Britton WJ. (2002): Autocrine IL 10 impairs dendritic cell (DC) derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph Biomedical Studies and Clinical Evaluations Winsome Publishing LLC @ 2024 Dubale Beyene 13 nodes and local IL 12 production. Eur J Immunol, 32:994 1002. [PubMed: 11920565].
Publisher | Google Scholor -
Dijkman, K., Aguilo, N., and Boot, C. (2021): Pulmonary MTBVAC vaccination induces immune signatures previously correlated with prevention of tuberculosis infection. Cell Reports Med, 2:100187.
Publisher | Google Scholor -
Dorman, SE., Holland, SM. (1998): Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J. Clin. Invest, 101: 2364–9.
Publisher | Google Scholor -
Du Toit, LC., Pillay, V., Danckwerts, MP. (2006): Tuberculosis chemotherapy: current drug delivery approaches. Respir Res, 7(1): 118
Publisher | Google Scholor -
Dye, C., Glaziou, P., Floyd, K., Raviglione, and M. (2013): Prospects for tuberculosis elimination. Annu Rev Public Health, 34:271-86.
Publisher | Google Scholor -
Edwards, KM. (2001): Development, Acceptance, and Use of Immunologic Correlates of Protection in Monitoring the Effectiveness of Combination Vaccines. Clin Infect Dis, 33 (4): S276-S279
Publisher | Google Scholor -
Encinales, L., Zuñiga, J., Granados Montiel, J., Yunis, M., Granados, J., Almeciga, I. (2010): Humoral immunity in tuberculin skin test anergy and its role in high risk persons exposed to active tuberculosis. Mol Immunol, 47:1066 73. doi: 10.1016/j.molimm.2009.11.005.
Publisher | Google Scholor -
Esmail, H., Riou, C., Du, Bruyn, E., Lai, R J., Harley, YXR., Meintjes, G., Wilkinson, KA., Wilkinson, RJ. (2018): The immune response to Mycobacterium tuberculosis in HIV 1 coinfected persons. Annu Rev Immunol, 36:603 638.
Publisher | Google Scholor -
Esparza, M., Palomares, B., Garcı´a T. (2015): PstS 1, the 38 kDa mycobacterium tuberculosis glycoprotein, is an adhesin, which binds the macrophage mannose receptor and promotes phagocytosis. Scand J Immunol, 81:46–55.
--> -
F.J. Roca. and L. Ramakrishnan. (2013): TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell, 153, pp. 521-534.
Publisher | Google Scholor -
Feris, EJ., Encinales, L., Awad, C., Stern, JN., Tabansky, I., Jiménez Alvarez L. (2016): High levels of anti tuberculin (IgG) antibodies correlate with the blocking of T cell proliferation in individuals with high exposure to Mycobacterium tuberculosis. Int J Infect Dis, 43:21–4. DOI: 10.1016/j.ijid.2015. 12.004.
--> -
Fletcher, HA., Snowden, MA., Landry, B., Rida, W., Satti, I., Harris, SA., et al. (2016): T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun, 7: 11290.
Publisher | Google Scholor -
Flynn, JL.and Chan, J. (2001): Immunology of tuberculosis. Annu Rev Immunol., 19: 93–129.
Publisher | Google Scholor -
Frieden, TR., Sterling, TR., Munsiff, SS., Watt, CJ. Dye, C. (2003): Tuberculosis. Lancet, 362(9387): 887-899.
--> -
Geldmacher, C., Ngwenyama, N., Schuetz, A., Petrovic, C., Reither, K., et al. (2010): Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med, 207:2869 –2881.
Publisher | Google Scholor -
Georgiou, G., Ippolito, GC., Beausang, J., Busse, CE., Wardemann, H., Quake, SR. (2014): The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat Biotechnol, 32:158-68.
Publisher | Google Scholor -
Glatman Freedman A, Casadevall A, Dai Z, Jacobs Jr WR, Li A, Morris SL, (2004): Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J Clin Microbiol, 42: 3225–31.
Publisher | Google Scholor -
Greenaway, C., Lienhardt, C., Adegbola, R. (2005): Humoral response to Mycobacterium tuberculosis antigens in patients with tuberculosis in the Gambia. Int J Tuberc Lung Dis, 9: 1112–19
Publisher | Google Scholor -
Gupta, RK., Lawn, SD., Bekker, L-G., Caldwell, J., Kaplan, R., Wood, R. (2013): Impact of human immunodeficiency virus and CD4 count on tuberculosis diagnosis: analysis of city-wide data from Cape Town, South Africa. Int J Tuberc Lung Dis, 17:1014-22.
Publisher | Google Scholor -
Gupta, RK., Lucas, SB., Fielding, KL., Lawn, SD. (2015): Prevalence of tuberculosis in post mortem studies of HIV infected adults and children in resource limited settings: a systematic review and meta analysands 29:1987 2002.
--> -
Hamasur, B., Haile, M., Pawlowski, A., Schroder, U., Williams, A., Hatch, G., Hall, G., Marsh, P., Kallenius, G., Svenson, SB. (2003): Mycobacterium tuberculosis arabinomannaneprotein conjugates protect against tuberculosis. Vaccine, 21: 4081-93.
--> -
Hawn, TR., Day, TA., Scribam, TJ., Hatherill, M., Hanekom, WA., Evans, TG., Churchyard, GJ., Kublin, JG., Bekker, L., Self, SG. (2003): Tuberculosis vaccines and prevention of infection. Microbiol Mol Biol Rev, 78:650-71.
Publisher | Google Scholor -
Hermann, C., Karamchand, L., Blackburn, JM. (2021): Cell envelope proteomics of mycobacteria. J Proteome Res, 20: 94–109. doi:10.1021/acs.jproteome.0c00650.
Publisher | Google Scholor -
Hogarth, PM. and Pietersz, GA. (2012): Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat Rev Drug Discov, 11:311 31. doi: 10.1038/nrd2909.
Publisher | Google Scholor -
Holt, P., Haining, S., Nelson, D., Sedgwick, J. (1994): Origin and steady-state turnover of class II MHC bearing dendritic cells in the epithelium of Biomedical Studies and Clinical Evaluations Winsome Publishing LLC @ 2024 Dubale Beyene 14 the conducting airways. J Immunol, 153:256–61. [PubMed: 8207240].
--> -
Hussain, R., Shiratsuchim, H., Phillips, M., Ellner, J., Wallis, RS. (2001): Opsonizing antibodies (IgG1) up-regulate monocyte proinflammatory cytokines tumor necrosis factor-alpha(TNF- alpha) and IL-6 but not anti inflammatory cytokine IL-10 in mycobacterial antigen-stimulated monocytes implications for pathogenesis. Clin Exp Immunol, 123:2108.
--> -
Inaba, K., Inaba, M., Naito, M., Steinman, R. (1993): Dendritic cell progenitors phagocytose particulates, including Bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med, 178:479–88. PubMed: 7688024.
Publisher | Google Scholor -
J.U, Igietseme., F.O. Eko. and Q. He., C.M. Black. (2004): Antibody regulation of Tcell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines, 3, Pp. 23-34.
Publisher | Google Scholor -
Joaquin, Zuniga., Diana, TorresGarcıa, Teresa., SantosMendoza, Tatiana., S., Rodriguez-Reyna, Julio., Granados Edmond J, Yunis. (2012): Cellular and Humoral Mechanisms Involved in the Control of Tuberculosis. Hindawi Publishing Corporation Clinical and Developmental Immunology Volume,
Publisher | Google Scholor -
Joller, N., Weber, SS., Muller, AJ., Sporri, R., Selchow, P., Sander P. (2010): Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting. The USA. Proc Natl Acad Sci,
Publisher | Google Scholor -
Joosten, SA., Fletcher, HA., Ottenhoff, THM. (2013): A helicopter perspective on TB biomarkers: pathway process-based based analysis of gene expression data provides new insight into TB pathogenesis. PLoS One, 8.
Publisher | Google Scholor -
Jouanguy, E., Lamhamedi-Cherradi, S., Altare, F. (1997): Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J. Clin. Invest, 100: 2658–64
Publisher | Google Scholor -
K.D. Mayer-Barber., B.B. Andrade., D.L. Barber., S. Hieny., C.G. Feng., P. Caspar., S. Oland., S. Gordon., A. Sher. (2011): Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection Immunity, 35, pp. 1023-1034
Publisher | Google Scholor -
Kaforou, M., Wright, VJ., Oni, T.,French, N., Anderson, ST., Bangani, N., et al. (2013): Detection of tuberculosis in HIV-infected and uninfected African adults using whole blood RNA expression signatures: a case control study. PLoS Med, 10:e1001538.
Publisher | Google Scholor -
Kaufmann, SHE., Lange, C., Rao, M., Balaji, KN., Lotze, M., Schito, M., Zumla, AI., Maeurer, M. (2014): Progress in tuberculosis vaccine development and host-directed therapies state of the art review. Lancet Respir Med, 2:301-20.
Publisher | Google Scholor -
Kawahara, JY., Irvine, EB., Alter, G. (2019): A case for antibodies as mechanistic correlates of immunity in tuberculosis. Front Immunol, 10:996. DOI: 10.3389/fimmu.2019.00996.
Publisher | Google Scholor -
Khader, S., Partida-Sanchez, S., Bell, G., Jelley-Gibbs, D., Swain, and S. (2006): Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med, 203:1805-15. PubMed: 16818672.
Publisher | Google Scholor -
Kimura, SG., Andia Biraro, I., Egesa, M.,Bagaya, BS., Raynes, JG., Levin, J. (2017): Use of QuantiFERON R-TB Gold in-tube culture supernatants for measurement of antibody responses. PLoS ONE. 12:e0188396. DOI: 10.1371/journal.pone.0188396.
--> -
Knechel, NA. (2009): Tuberculosis: pathophysiology, clinical features, and diagnosis. Critical Care Nurse, 29(2): 34-43.
Publisher | Google Scholor -
KunnathVelayudhan, S., Davidow, AL., Wang, HY., Molina, DM., Huynh, VT., Salamon, H., Pine, R., Michel, G., Perkins, MD., Xiaowu, L., Felgner, PL., Flynn, JL., Catanzaro, A., Gennaro, ML. (2012). Proteome scale antibody responses and outcome of Mycobacterium tuberculosis infection in nonhuman primates and tuberculosis patients. J Infect Dis, 206:697-705.
Publisher | Google Scholor -
L. Ye, R. Zeng, Y. Bai, D. C. Roopenian, and X. Zhu. (2011): Efficient mucosal vaccination mediated by the neonatal Fc receptor,” Nature Biotechnology, vol. 29, no. 2, pp. 158–163.
Publisher | Google Scholor -
Laal, S., Saanich, KM., Sonnenberg, MG. (1997): Surrogate marker of preclinical tuberculosis in human immunodeficiency virus infection: antibodies to an 88-kDa secreted antigen of Mycobacterium tuberculosis. J Infect Dis, 176:133–43.
--> -
Le Thuy, N., Borrero, R., Férnandez, S., Reyes, G., Perez, JL., et al. (2010): Evaluation of the potential of Mycobacterium smegmatis as vaccine Candidate against tuberculosis by in silico and in vivo studies. VacciMonitor, 19 (1):20-6.
Publisher | Google Scholor -
Lee, Kozakiewicz., Jiayao, Phuah., JoAnne, Flynn., John, Chan. (2013): The Role of B Cells and Humoral Immunity in Mycobacterium tuberculosis Infection M. Divangahi (Ed.). The New Paradigm of Immunity to Tuberculosis Advances in Experimental Medicine and Biology,
--> -
Lehar, SM., Pillow, T., Xu, M., Staben, L., Kajihara, KK., Vandlen, R., DePalatis, L., Raab, H., Hazenbos, WL., Morisaki, JH. (2015): Novel antibody antibiotic conjugate eliminates intracellular S. aureus. Nature, 527:323-8.
Publisher | Google Scholor -
Lenette, L., L Amy, W., ChungTracy, R., RosebrockMusice., Ghebremichael, Wen., Hanyuu, Patricia S., et al. (2016): A Functional Role for Antibodies in Tuberculosis, Pp 433-443.e14.
--> -
Li, H., Wang, XX., Wang, B., Fu, L., Liu, G., Lu, Y. (2017): Latently and uninfected health care workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc Natl Acad Sci, 114:5023-8.
Publisher | Google Scholor -
Lin, PL., Rutledge, T., Green, AM., Bigbee, M., Fuhrman, C., Klein, E., Flynn, JL. (2012): CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on the severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses 28:1693 1702.
Publisher | Google Scholor -
Locht, C., Hougardy, JM., Rouanet, C. (2006): Heparin-binding hemagglutinin, from an extrapulmonary dissemination factor to a powerful diagnostic and protective antigen against tuberculosis. Tuberculosis, 86 303–09.
Publisher | Google Scholor -
Lu, LL., Smith, MT., Krystle, K., Luedemann, C., Suscovich, TJ., Grace, PS. (2019): IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat Med, 25:977–87. DOI: 10.1038/s41591-019-0441-3.
Publisher | Google Scholor -
Lu, LL., Chung, AW., Rosebrock, TR., Ghebremichael, M., Yu, WH., Grace, PS. (2016): A functional role for antibodies in tuberculosis. Cell, 167:433-43.414. DOI: 10.1016/j.cell.2016.08.072
--> -
Lubbers, R., Sutherland, JS., Goletti, D., De Paus, RA., Van Moorsel, CHM., Veltkamp, M. (2018): Complement component C1q as serum biomarker to detect active tuberculosis.
Publisher | Google Scholor -
Lux, S., Aschermann, M., Biburger, F, Nimmerjahn. (2010): The pro and anti-inflammatory activities of immunoglobulin G, Ann. Rheum. Dis, 69 (Suppl1) Pp. i92-i96
Publisher | Google Scholor -
Lyashchenko, K., Colangeli, R., Houde, M., Al Jahdali, H., Menzies, D., Gennaro, ML. (1998): Heterogeneous antibody responses in tuberculosis. Infect Immun, 66: 3936e40. Front Immunol, 9:2427. DOI: 10.3389/fimmu.2018.02427.
--> -
M, Ouimet., S, Koster., E, Sakowski., B, Ramkhelawon., C, van Solingen., S, Oldebeken., D, Karunakaran., C, Portal Celhay., F.J, Sheedy., T.D, Ray. (2016): Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol, 17, Pp. 677-686.
Publisher | Google Scholor -
M. Suga., F. Tanaka., H. Muranaka., H. Nishikawa., M, Ando (1996): Effect of antibacterial antibody on bactericidal activities of superoxide and lysosomal enzyme from alveolar macrophages in rabbits. Respirology, 1, Pp. 127-132.
Publisher | Google Scholor -
M.D. Mossalayi., I. Vouldoukis., M. Mamani-Matsuda., T. Kauss, J. Guillon., J. Maugein., D. Moynet., J. Rambert., V, Despla., D, Mazier. (2009): CD23 mediates the antimycobacterial activity of human macrophages. Infect Immun, 77, Pp. 5537-5542.
Publisher | Google Scholor -
M.D, Tameris., M, Hatherill., B.S, Landry., T.J, Scriba., M.A, Snowden., S, Lockhart., J.E, Shea., B.J, Bruce., G.D, Hussey., W.A, Hanekom, H., Mahomed, H., McShane. (2013): Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCGrandomizedised, placebo-controlled phase 2b trial Lancet, 381 pp. 1021-1028, (13)60177-4
--> -
Maes, RF. (1991): Evaluation of the avidity of IgG anti-mycobacterial antibodies in tuberculous patient’s serum by an A-60 immunoassay. Eur J Epidemiol, 7:188 190.
Publisher | Google Scholor -
Mawuenyega, KG., Forst, CV., Dobos, KM. (2005): Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Mol Biol Cell, 16(1): 398-404.
Publisher | Google Scholor -
Milla, R., McLean Lenette, L., Lu Stephen, J., Kent, Amy., W, Chung. (2019): An Inflammatory Story: Antibodies in Tuberculosis Comorbidities.doi:10.3389/fimmu.2019.02846
--> -
Moh, ES., Lin, CH., Thaysen Andersen, M., Packer, NH. (2016): Site-specific N-glycosylation of recombinant pentameric and hexameric-human IgM. J Am Soc Mass Spectrom, 27:1143–55. DOI: 10.1007/s13361-016-1378-0.
Publisher | Google Scholor -
Morris, K. (2011): WHO recommends against inaccurate tuberculosis tests. The Lancet, 379(9982): 113-4.
Publisher | Google Scholor -
Nabeshima, S, Murata, M., Kashiwagi, K. (2005): Serum antibody response to tuberculosis associated glycolipid antigen after BCG vaccination in adults. J Infect Chemother, 11: 256–58.
Publisher | Google Scholor -
Navona, JA., Laal, S., Pirofski, L., McLean, G., Robbins, JB. (2003): Specificity and diversity of antibodies to Mycobacterium tuberculosis arabinomannan. Clin Diagn Lab Immunol, 10:90-96.
--> -
Ndiaye, BP., Thienemann, F., Ota, M., Landry, BS., Camara, M., et al. (2015): Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomized, placebo-controlled, phase 2 trial. Biomedical Studies and Clinical Evaluations Winsome Publishing LLC @ 2024 Dubale Beyene 16 Lancet Respir Med, 3:190-200. S2213-2600(15)00037-5.
Publisher | Google Scholor -
Newport MJ, Huxley CM, Huston S (1996): A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med, 335: 1941–9.
Publisher | Google Scholor -
Norazmi, MN., Sarmiento, ME., Acosta, A. (2005): Recent advances in tuberculosis vaccine development. Curr Resp Med Rev, 1(12):109-16.
Publisher | Google Scholor -
Noss, EH., Pai, RK., Sellati, TJ. (2001): Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol, 167:910–18.
Publisher | Google Scholor -
Nunes-Alves, C., Booty, MG., Carpenter, SM., Jayaraman. P., Rothchild, AC., Behar, SM. (2014): In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol, 12:289e99
--> -
O’Toole, R., Smeulders, MJ., Blokpoel, MC. (2003): A two-component regulator of universal stress protein expression and adaptation to oxygen starvation in Mycobacterium smegmatis. J Bacteriol, 185:1543–54.
Publisher | Google Scholor -
Orme, IM. (2014): Vaccines to prevent tuberculosis infection rather than the disease: physiological and immunological aspects. Tuberculosis: 17.
Publisher | Google Scholor -
Ottenhoff, T. (2012): The knowns and unknowns of the immunopathogenesis of tuberculosis. Int J Tuberc Lung Dis, 16:1424–32. DOI: 10.5588/ijtld.12.0479.
Publisher | Google Scholor -
P, Pancholi., A, Mirza., N, Bhardwaj., R.M, Steinman. (1993): Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages Science, 260, pp. 984-986.
Publisher | Google Scholor -
P. L. Ogra, H., Faden, and R. C. Welliver. (2001): Vaccination strategies for mucosal immune responses, Clinical Microbiology Reviews, 14(2), pp. 430–445.
Publisher | Google Scholor -
Pagan, JD., Kitaoka, M., Anthony, RM. (2018): Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell, 172:564– 77.e513. DOI: 10.1016/j.cell.2017.11.041.
Publisher | Google Scholor -
Paulson T. (2013): Epidemiology: a mortal foe. Nature. 502:S2–3. DOI: 10.1038/502S2a
Publisher | Google Scholor -
Perley, CC., Frahm, M., Click, EM, Dobos, KM, Ferrari, G., Stout JE, Frothingham, R. (2014): The human antibody response to the surface of Mycobacterium tuberculosis. PLoS One 9:e98938.
--> -
Pethe, K., Alonso, S., Biet, F. (2001): The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature, 412:190–94.
Publisher | Google Scholor -
Plotkin, SA. (2008): Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis, 47:401e9.
Publisher | Google Scholor -
Pollard, AJ., Perrett, KP., Beverley, PC. (2009): Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol, (3):213-20.
Publisher | Google Scholor -
Rao, M., Valentini, D., Poiret, T., Dodoo, E., Parida, S., Zumla, A., Brighenti, S., Maeurer, M. (2015): B cells as mediators of clinically relevant immune responses in tuber
Publisher | Google Scholor -
Rodríguez, L., Tirado, Y., Reyes, F. (2011): Proteoliposomes from Mycobacterium smegmatis induce immune cross-reactivity against Mycobacterium tuberculosis antigens in mice. Vaccine; 29(37):6436-41.
Publisher | Google Scholor -
Roy, A., Eisenhut, M., Harris, RJ., Rodrigues, LC., Sridhar, S., Habermann, S., Snell, L., Mangtani, P., Adetifa, I., Lalvani, A., Abubakar I. (2014): Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ, 349: g4643.
Publisher | Google Scholor -
Roy, Chowdhury., R, Vallania., F, Yang., Q. (2006): Lop associated with M. tuberculosis infection outcomes. Nature, 560: 644– 8. DOI: 10.1038/s41586-018-0439-x.
--> -
Schwebach, JR., Glatman-Freedman, A., Gunter Cummins, L., Dai, Z., Robbins, JR., Schneerson, R. (2002): Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo. Infect Immune, 72: 2578–77.
Publisher | Google Scholor -
Scriba, TJ., Coussens, AK., Fletcher, HA. (2017): Human immunology of tuberculosis. Microbiol Spectrum, 5(1): TBTB2-0016- 2016. DOI: 10.1128/microbiolspec.TBTB2-0016-2016.
Publisher | Google Scholor -
Seder, RA., Hill, AV. (2000): Vaccines against intracellular infections requiring cellular immunity. Nature, 406(6797):793–798.
--> -
Simmons, JD., Stein, CM., Seshadri, C., Campo, M., Alter, G., Fortune, S. (2018): Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol, 18:575–89. DOI: 10.1038/s41577-018-0025-3.
Publisher | Google Scholor -
Steingart, KR., Dendukuri, N., Henry M. (2009): Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol, 16:260–76.
Publisher | Google Scholor -
Sutherland, JS., Loxton, AG., Haks, MC., Kassa, D., Ambrose, L., Lee, JS., et a;. (2014): Biomarkers for TB consortium. Differential gene expression of activating Fcℽ receptor classifies active tuberculosis regardless of human immunodeficiency virus status or ethnicity. Clin Microbiol Infect, 20: O230–8. DOI: 10.1111/1469-0691.12383.
Publisher | Google Scholor -
T. H. Mogensen. (2009): Pathogen recognition and inflammatory signaling in innate immune defenses, Clinical Microbiology Reviews, 22(2), pp. 240– 273.
Publisher | Google Scholor -
Tamaris, MD., Hatherill, M., Landry, BS., Scriba, TJ., Snowden, MA., Lockhart, S., Shea, JE., Bruce, BJ., Hussey, GD., Hanekom, WA., Mahomed, H., McShane, H. (2013): Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomized, placebo-controlled phase 2b trial. Lancet, 381:1021e8. (13)60177-4.
--> -
Thiem, VD., Lin, FY., Canh, do G., Son, NH., Anh, DD., Mao, ND., Chu, C., Hunt, SW., Robbins, JB., Schneerson, R., Szu, SC. (2011): The Vi conjugate typhoid vaccine is safe, elicits protective levels of IgG anti-Vi, and is compatible with routine infant vaccines. Clin Vaccine Immunol, (5):750-5
Publisher | Google Scholor -
Tjarnlund, A., Rodríguez, A., Cardona, P-J., Guirado, E., Ivanyi, J., Singh, M., Troye, Blomberg M., Fernandez, C. (2006): Polymeric IgR knockout mice are more susceptible to mycobacterial infections in the respiratory tract than wild-type mice. Int Immunol, 18:807-16.
Publisher | Google Scholor -
Trunz, B., Fine, P., Dye, C. (2006): Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a metaanalysis and assessment of cost-effectiveness. Lancet, 367:1173–80. [PubMed: 16616560].
Publisher | Google Scholor -
Van Crevel, R., Ottenhoff, TH., Vander Meer, JW. (2002): Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev, 15(2): 294-309.
Publisher | Google Scholor -
Velayudhan, SK, and Gennaro, ML. (2010): Antibody responses in tuberculosis. In: Norazmi MN, Acosta A, Sarmiento ME, Eds. The Art &Science of tuberculosis vaccine development. 1st ed. Malaysia. Oxford University Press; p.188-208.
Publisher | Google Scholor -
Velmurugan, B, Chen, J.L., Miller, S., Azogue, S., Gurses, T., Hsu, M., Glickman, W.R., Jacobs Jr., S.A. Porcelli, V., Briken. (2007): Mycobacterium tuberculosis is not isis an opulence gene that inhibits apoptosis of infected host cells. PLoS Pathog, 3, p. 110.
--> -
Weijie, Zhai., Fengjuan, Wu., Yiyuan , Zhang. , Yurong, Fu .and Zhijun, Liu. (2019): The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int. J. Mol. Sci, 20, 340; DOI: 10.3390/ijms20020340.
Publisher | Google Scholor -
Wolf, AJ., Linas, B., Trevejo Nunez, GJ., Kincaid, E., Tamura, and T. (2007): Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol, 179:2509–19. PubMed: 17675513.
--> -
World Health Organization. Global Tuberculosis Report 2019. Geneva. (2019).
Publisher | Google Scholor -
World Health Organization. Global tuberculosis report. (2015): p. 2015.
Publisher | Google Scholor -
World Health Organization. (2013): Global tuberculosis report 2013.
--> -
Yao S, Huang D, Chen CY, Halliday L, Wang RC, Chen ZW. (2014): CD4 T cells contain earlyextrapulmonaryy tuberculosis (TB) dissemination and rapid TB progression and sustain multi effector functions of CD8 T and CD3 lymphocytes: mechanism of CD4 T cell immunity. J Immunol, 192:2120-2132.
Publisher | Google Scholor -
Z.A. Malik., G.M. Denning., DJ, Kusner. (2000): Inhibition of Ca (2+) signaling by Mycobacterium tuberculosis is associated with reduced phagosomelysosome fusion and increased survival within human macrophages. J. Exp. Med, 191 Pp. 287- 302.
Publisher | Google Scholor -
Zimmermann, N., Thormann, V., Hu, B., Kohler, AB., Imai-Matsushima, A., Locht, C. (2016): Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol Med, 8:1325-39.DOI: 10.15252/emmm.201606330.
Publisher | Google Scholor